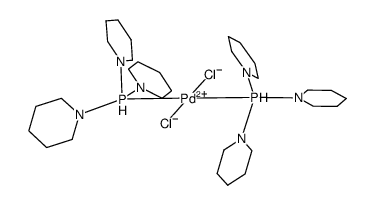
(2,6-Bis((di(piperidin-1-yl)phosphino)amino)phenyl)palladium(II) chloride

(2,6-Bis((di(piperidin-1-yl)phosphino)amino)phenyl)palladium(II) chloride structure
|
Common Name | (2,6-Bis((di(piperidin-1-yl)phosphino)amino)phenyl)palladium(II) chloride | ||
|---|---|---|---|---|
| CAS Number | 955035-37-7 | Molecular Weight | 645.49600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C26H45ClN6P2Pd | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | (2,6-Bis((di(piperidin-1-yl)phosphino)amino)phenyl)palladium(II) chloride |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | C26H45ClN6P2Pd |
|---|---|
| Molecular Weight | 645.49600 |
| Exact Mass | 644.19000 |
| PSA | 64.20000 |
| LogP | 3.60960 |
| InChIKey | XASCRSOQTJTSCW-UHFFFAOYSA-M |
| SMILES | [Cl-].[Pd+2].[c-]1c(NP(N2CCCCC2)N2CCCCC2)cccc1NP(N1CCCCC1)N1CCCCC1 |
|
~% 
(2,6-Bis((di(pi... CAS#:955035-37-7 |
| Literature: Bolliger, Jeanne L.; Blacque, Olivier; Frech, Christian M. Angewandte Chemie - International Edition, 2007 , vol. 46, # 34 p. 6514 - 6517 |
|
Comparative Study of the Frech Catalyst with Two Conventional Catalysts in the Heck Synthesis of 2,4-Diaminopyrimidine-based Antibiotics.
Org. Prep. Proced. Int. 45(1) , 66-71, (2013)
|
|
|
Short, facile, and high-yielding synthesis of extremely efficient pincer-type suzuki catalysts bearing aminophosphine substituents.
Angew. Chem. Int. Ed. Engl. 46 , 6514-6517, (2007)
|
|
|
Rationally designed pincer-type heck catalysts bearing aminophosphine substituents: Pd IV intermediates and palladium nanoparticles.
Chemistry 14 , 7969-7977, (2008) The aminophosphine-based pincer complexes [C6H3-2,6-(XP(piperidinyl)2)2Pd(Cl)] (X=NH 1; X=O 2) are readily prepared from cheap starting materials by sequential addition of 1,1',1''-phosphinetriyltripi... |
| 1-N,3-N-bis[di(piperidin-1-yl)phosphanyl]benzene-2-ide-1,3-diamine,chloropalladium(1+) |