N-Methylformanilide
Modify Date: 2025-08-21 07:06:12
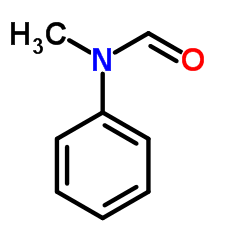
N-Methylformanilide structure
|
Common Name | N-Methylformanilide | ||
|---|---|---|---|---|
| CAS Number | 93-61-8 | Molecular Weight | 135.163 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 243.0±9.0 °C at 760 mmHg | |
| Molecular Formula | C8H9NO | Melting Point | 8-13 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 126.7±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | N-Methylformanilide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 243.0±9.0 °C at 760 mmHg |
| Melting Point | 8-13 °C(lit.) |
| Molecular Formula | C8H9NO |
| Molecular Weight | 135.163 |
| Flash Point | 126.7±0.0 °C |
| Exact Mass | 135.068420 |
| PSA | 20.31000 |
| LogP | 1.09 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.567 |
| InChIKey | JIKUXBYRTXDNIY-UHFFFAOYSA-N |
| SMILES | CN(C=O)c1ccccc1 |
| Water Solubility | immiscible |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R38 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| HS Code | 29242995 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Redox inactive metal ion triggered N-dealkylation by an iron catalyst with dioxygen activation: a lesson from lipoxygenases.
Dalton Trans. 44 , 9847-59, (2015) Utilization of dioxygen as the terminal oxidant at ambient temperature is always a challenge in redox chemistry, because it is hard to oxidize a stable redox metal ion like iron(III) to its high oxida... |
| Formanilide, N-methyl- |
| FORMAMIDE,N-METHYL-N-PHENYL- |
| N-Phenyl-N-methylformamide |
| N-METHYL-N-FORMANILIDE |
| N-Methyl-N-formylaniline |
| N-METHYLFORMANILID |
| Formamide, N-methyl-N-phenyl- |
| N-METHYLFORMANILIDE FOR SYNTHESIS |
| EINECS 202-262-3 |
| N-Methylformanilide |
| N-METHYL FORMANILIDE |
| methyl(phenyl)formamide |
| MFCD00003283 |
| N-Methyl-N-phenylformamide |
| N-methyl-N-benzamide |
| N-Formyl-N-methylaniline |
 CAS#:64-18-6
CAS#:64-18-6 CAS#:100-61-8
CAS#:100-61-8 CAS#:77287-34-4
CAS#:77287-34-4 CAS#:149-73-5
CAS#:149-73-5 CAS#:103-70-8
CAS#:103-70-8 CAS#:74-88-4
CAS#:74-88-4 CAS#:66-25-1
CAS#:66-25-1 CAS#:121-69-7
CAS#:121-69-7 CAS#:67-66-3
CAS#:67-66-3![5-CHLORO-4-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE structure](https://image.chemsrc.com/caspic/488/104804-16-2.png) CAS#:104804-16-2
CAS#:104804-16-2 CAS#:2538-98-9
CAS#:2538-98-9 CAS#:34123-66-5
CAS#:34123-66-5 CAS#:127595-56-6
CAS#:127595-56-6 CAS#:3392-97-0
CAS#:3392-97-0 CAS#:35250-76-1
CAS#:35250-76-1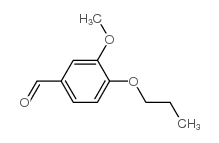 CAS#:57695-98-4
CAS#:57695-98-4 CAS#:5922-56-5
CAS#:5922-56-5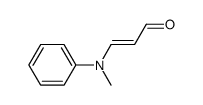 CAS#:34900-01-1
CAS#:34900-01-1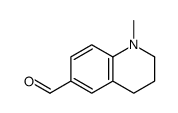 CAS#:493-50-5
CAS#:493-50-5
