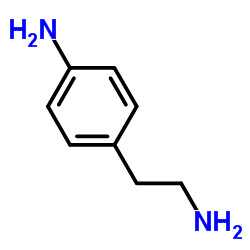
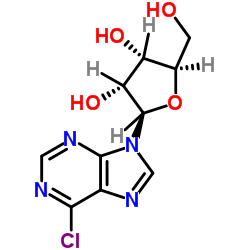
N6-[2-(4-Aminophenyl)ethyl]adenosine
![N6-[2-(4-Aminophenyl)ethyl]adenosine Structure](https://image.chemsrc.com/caspic/201/89705-21-5.png)
N6-[2-(4-Aminophenyl)ethyl]adenosine structure
|
Common Name | N6-[2-(4-Aminophenyl)ethyl]adenosine | ||
|---|---|---|---|---|
| CAS Number | 89705-21-5 | Molecular Weight | 386.40500 | |
| Density | 1.65g/cm3 | Boiling Point | 781.8ºC at 760 mmHg | |
| Molecular Formula | C18H22N6O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 426.6ºC | |
Use of N6-[2-(4-Aminophenyl)ethyl]adenosineN6-[2-(4-Aminophenyl)ethyl]adenosine is a potent, non-selective A3 adenosine receptor agonist.Target: Adenosine receptor agonist.in vitro: N6-[2-(4-Aminophenyl)ethyl]adenosine is a non-selective agonist of the adenosine A3 receptors, at the subprotective dose of 1 mg/kg against electroconvulsions, significantly potentiates the anticonvulsive action of phenobarbital, diphenylhydantoin and valproate against maximal electroshock, being ineffective at lower doses. N6-[2-(4-Aminophenyl)ethyl]adenosine (0.0039-1 mg/kg) also enhances the protective activity of carbamazepine. N6-[2-(4-Aminophenyl)ethyl]adenosine at low doses potentiates the protective activity of Carbamazepine most likely through the A subtype of adenosine receptors. At higher doses, N6-[2-(4-Aminophenyl)ethyl]adenosine seems to enhance the anticonvulsive effect of other antiepileptics via adenosine A1 receptors. [1]in vivo: N6-[2-(4-Aminophenyl)ethyl]adenosine (2-4 mg/kg) has no significant effect on seizure parameters (seizure severity, seizure duration and afterdischarge duration) in amygdala-kindled rats. N6-[2-(4-Aminophenyl)ethyl]adenosine is combined with antiepileptic drugs administered at doses ineffective in fully kindled rats.[2] |
| Name | (2R,3R,4S,5R)-2-[6-[2-(4-aminophenyl)ethylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol |
|---|---|
| Synonym | More Synonyms |
| Description | N6-[2-(4-Aminophenyl)ethyl]adenosine is a potent, non-selective A3 adenosine receptor agonist.Target: Adenosine receptor agonist.in vitro: N6-[2-(4-Aminophenyl)ethyl]adenosine is a non-selective agonist of the adenosine A3 receptors, at the subprotective dose of 1 mg/kg against electroconvulsions, significantly potentiates the anticonvulsive action of phenobarbital, diphenylhydantoin and valproate against maximal electroshock, being ineffective at lower doses. N6-[2-(4-Aminophenyl)ethyl]adenosine (0.0039-1 mg/kg) also enhances the protective activity of carbamazepine. N6-[2-(4-Aminophenyl)ethyl]adenosine at low doses potentiates the protective activity of Carbamazepine most likely through the A subtype of adenosine receptors. At higher doses, N6-[2-(4-Aminophenyl)ethyl]adenosine seems to enhance the anticonvulsive effect of other antiepileptics via adenosine A1 receptors. [1]in vivo: N6-[2-(4-Aminophenyl)ethyl]adenosine (2-4 mg/kg) has no significant effect on seizure parameters (seizure severity, seizure duration and afterdischarge duration) in amygdala-kindled rats. N6-[2-(4-Aminophenyl)ethyl]adenosine is combined with antiepileptic drugs administered at doses ineffective in fully kindled rats.[2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.65g/cm3 |
|---|---|
| Boiling Point | 781.8ºC at 760 mmHg |
| Molecular Formula | C18H22N6O4 |
| Molecular Weight | 386.40500 |
| Flash Point | 426.6ºC |
| Exact Mass | 386.17000 |
| PSA | 151.57000 |
| LogP | 0.32880 |
| Index of Refraction | 1.775 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~% ![N6-[2-(4-Aminophenyl)ethyl]adenosine Structure](https://image.chemsrc.com/caspic/201/89705-21-5.png)
N6-[2-(4-Aminop... CAS#:89705-21-5 |
| Literature: Bressi; Choe; HoughHough; Buckner; Van Voorhis; Verlinde; Hol; Gelb Journal of Medicinal Chemistry, 2000 , vol. 43, # 22 p. 4135 - 4150 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Adenosine receptor subtypes.
Trends Pharmacol. Sci. 21 , 360-366, (1993) The numerous and widespread effects of adenosine provide both an opportunity for the development of novel therapeutic agents acting via adenosine receptors and the challenge of achieving selectivity o... |
|
|
Adenosine A3 receptors mediate hypotension in the angiotensin II-supported circulation of the pithed rat.
Br. J. Pharmacol. 109 , 3-5, (1993) The cardiovascular effects of N6-2-(4-aminophenyl)ethyladenosine (APNEA), which when radiolabelled with 125I shows high affinity for the newly described adenosine A3 receptor, have been investigated i... |
|
|
Adenosine A3 receptors: two into one won't go.
Trends Pharmacol. Sci. 14 , 290, (1993)
|
| apnea |
| N6-[2-(4-Aminophenyl)ethyl]adenosine |