Homophthalic acid
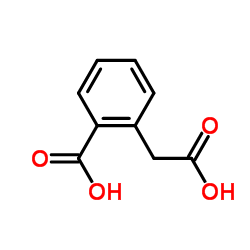
Homophthalic acid structure
|
Common Name | Homophthalic acid | ||
|---|---|---|---|---|
| CAS Number | 89-51-0 | Molecular Weight | 180.157 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 389.5±17.0 °C at 760 mmHg | |
| Molecular Formula | C9H8O4 | Melting Point | 178-182 °C(lit.) | |
| MSDS | USA | Flash Point | 203.5±17.4 °C | |
| Name | Homophthalic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 389.5±17.0 °C at 760 mmHg |
| Melting Point | 178-182 °C(lit.) |
| Molecular Formula | C9H8O4 |
| Molecular Weight | 180.157 |
| Flash Point | 203.5±17.4 °C |
| Exact Mass | 180.042252 |
| PSA | 74.60000 |
| LogP | 1.18 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.600 |
| InChIKey | ZHQLTKAVLJKSKR-UHFFFAOYSA-N |
| SMILES | O=C(O)Cc1ccccc1C(=O)O |
| Water Solubility | 12 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CY1575590 |
| HS Code | 2917399090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2917399090 |
|---|---|
| Summary | 2917399090 aromatic polycarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Catabolism of homophthalic acid by a soil bacterium.
FEMS Microbiol. Lett. 49(2-3) , 305-8, (1989) A microorganism capable of degrading homophthalic acid as a sole source of carbon was isolated from soil. The strain was tentatively identified as Pseudomonas sp. Oxygen uptake studies were carried ou... |
|
|
An expeditious and environmentally friendly synthesis of 3-substituted isocoumarins using microwave irradiation.
Nat. Prod. Res. 22(13) , 1120-7, (2008) A microwave-assisted, environmentally friendly, high-yielding, time-saving synthesis of medicinally important 3-substituted isocoumarins was carried out in a single step by direct condensation of homo... |
|
|
Metabolic pathway of homophthalic acid in Pseudomonas alcaligenes.
FEMS Microbiol. Lett. 110(1) , 59-64, (1993) A microorganism capable of degrading homophthalic acid as a sole carbon source was isolated from garden soil. The strain was identified as Pseudomonas alcaligenes. The organism degraded homophthalate ... |
| 2-(Carboxymethyl)benzoic acid |
| Benzeneacetic acid, 2-carboxy- |
| a-Carboxy-o-toluic Acid |
| MFCD00004326 |
| EINECS 201-913-9 |
| Homophthalic Acid |
 CAS#:95-13-6
CAS#:95-13-6 CAS#:201230-82-2
CAS#:201230-82-2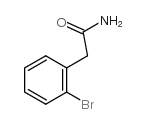 CAS#:65999-53-3
CAS#:65999-53-3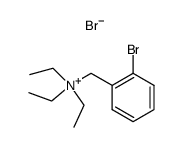 CAS#:85267-36-3
CAS#:85267-36-3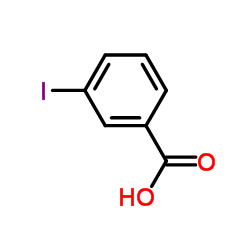 CAS#:88-67-5
CAS#:88-67-5 CAS#:141-97-9
CAS#:141-97-9 CAS#:100397-38-4
CAS#:100397-38-4 CAS#:703-59-3
CAS#:703-59-3 CAS#:528-46-1
CAS#:528-46-1 CAS#:101268-34-2
CAS#:101268-34-2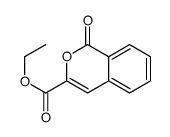 CAS#:111686-63-6
CAS#:111686-63-6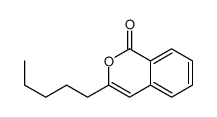 CAS#:106180-94-3
CAS#:106180-94-3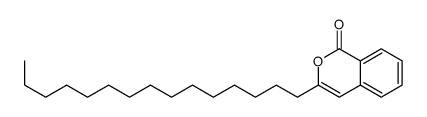 CAS#:106180-95-4
CAS#:106180-95-4 CAS#:34014-51-2
CAS#:34014-51-2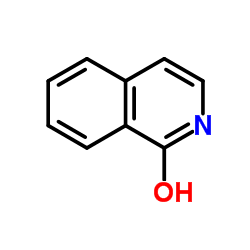 CAS#:491-30-5
CAS#:491-30-5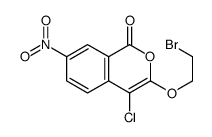 CAS#:141468-73-7
CAS#:141468-73-7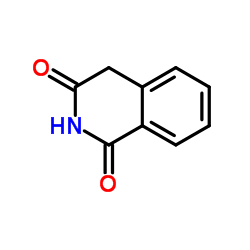 CAS#:4456-77-3
CAS#:4456-77-3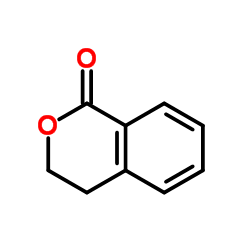 CAS#:4702-34-5
CAS#:4702-34-5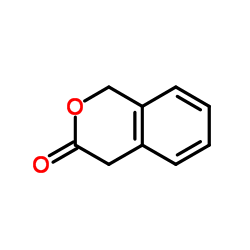 CAS#:4385-35-7
CAS#:4385-35-7 CAS#:493-05-0
CAS#:493-05-0
