Atorvastatin Epoxy Tetrahydrofuran Impurity
Modify Date: 2025-08-25 18:22:30
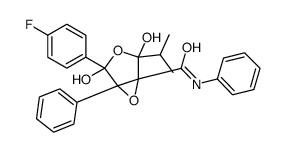
Atorvastatin Epoxy Tetrahydrofuran Impurity structure
|
Common Name | Atorvastatin Epoxy Tetrahydrofuran Impurity | ||
|---|---|---|---|---|
| CAS Number | 873950-19-7 | Molecular Weight | 449.47100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C26H24FNO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Atorvastatin Epoxy Tetrahydrofuran ImpurityAtorvastatin Epoxy Tetrahydrofuran Impurity is an impurity isolated oxidative degradation products of Atorvastatin (HY-B0589)[1]. Atorvastatin is an orally active HMG-CoA reductase inhibitor, has the ability to effectively decrease blood lipids. |
| Name | 4-(4-fluorophenyl)-2,4-dihydroxy-N,5-diphenyl-2-propan-2-yl-3,6-dioxabicyclo[3.1.0]hexane-1-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Atorvastatin Epoxy Tetrahydrofuran Impurity is an impurity isolated oxidative degradation products of Atorvastatin (HY-B0589)[1]. Atorvastatin is an orally active HMG-CoA reductase inhibitor, has the ability to effectively decrease blood lipids. |
|---|---|
| Related Catalog | |
| In Vitro | Atorvastatin treatment decreases apoptosis of myocardial cells by down-regulating GRP78, caspase-12 and CHOP expression in myocardial cells after myocardial infarction, and the endoplasmic reticulum (ER) stress is activated in response to heart failure and angiotensin II (Ang II) stimulation. |
| References |
| Molecular Formula | C26H24FNO5 |
|---|---|
| Molecular Weight | 449.47100 |
| Exact Mass | 449.16400 |
| PSA | 94.81000 |
| LogP | 4.29830 |
| Hazard Codes | Xi |
|---|
|
~% 
Atorvastatin Ep... CAS#:873950-19-7 |
| Literature: TEVA PHARMACEUTICAL INDUSTRIES LTD.; TEVA PHARMACEUTICALS USA, INC. Patent: WO2006/37125 A1, 2006 ; Location in patent: Page/Page column 17 ; |
| atorvastatin calcium epoxide dihydroxy |
| Atorvastatin Epoxy Tetrahydrofuran Impurity |
| ATV-epoxy furan |
| Atorvastatin Impurity 5 |