Di-tert-Butyl azodicarboxylate
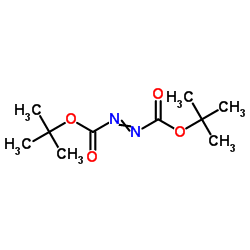
Di-tert-Butyl azodicarboxylate structure
|
Common Name | Di-tert-Butyl azodicarboxylate | ||
|---|---|---|---|---|
| CAS Number | 870-50-8 | Molecular Weight | 230.261 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 287.1±9.0 °C at 760 mmHg | |
| Molecular Formula | C10H18N2O4 | Melting Point | 89-92 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 107.2±13.2 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
| Name | Di-tert-Butyl azodicarboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 287.1±9.0 °C at 760 mmHg |
| Melting Point | 89-92 °C(lit.) |
| Molecular Formula | C10H18N2O4 |
| Molecular Weight | 230.261 |
| Flash Point | 107.2±13.2 °C |
| Exact Mass | 230.126663 |
| PSA | 77.32000 |
| LogP | 3.24 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.460 |
| Storage condition | 2-8°C |
| Water Solubility | Insoluble |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360FD |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | F:Flammable;Xi:Irritant; |
| Risk Phrases | R11;R36/37/38 |
| Safety Phrases | S26-S36-S37/39-S16 |
| RIDADR | 1325 |
| WGK Germany | 3 |
| RTECS | VZ2275000 |
| HS Code | 2927000090 |
|
~98% 
Di-tert-Butyl a... CAS#:870-50-8 |
| Literature: Juermann, Gea; Tsubrik, Olga; Tammeveski, Kaido; Maeeorg, Uno Journal of Chemical Research, 2005 , # 10 p. 661 - 662 |
|
~% 
Di-tert-Butyl a... CAS#:870-50-8 |
| Literature: Journal of the American Chemical Society, , vol. 134, # 45 p. 18581 - 18584 |
|
~% 
Di-tert-Butyl a... CAS#:870-50-8 |
| Literature: Tetrahedron Letters, , vol. 43, # 35 p. 6213 - 6215 |
| Precursor 3 | |
|---|---|
| DownStream 7 | |
| HS Code | 2927000090 |
|---|---|
| Summary | 2927000090 other diazo-, azo- or azoxy-compounds。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Axially chiral guanidine as highly active and enantioselective catalyst for electrophilic amination of unsymmetrically substituted 1,3-dicarbonyl compounds.
J. Am. Chem. Soc. 128 , 16044, (2006) A newly designed axially chiral guanidine is found to function as an effective platform for asymmetric induction at the alpha-carbon of unsymmetrically substituted 1,3-dicarbonyl compounds. Highly eff... |
|
|
Chirally aminated 2-naphthols--organocatalytic synthesis of non-biaryl atropisomers by asymmetric Friedel-Crafts amination.
Angew. Chem. Int. Ed. Engl. 45(7) , 1147-1151, (2006)
|
|
|
Catalytic Enantioselective Reaction of α-Aminoacetonitriles Using Chiral Bis(imidazoline) Palladium Catalysts.
Angew. Chem. Int. Ed. Engl. 54(28) , 8198-202, (2015) The catalytic enantioselective reaction of diphenylmethylidene-protected α-aminoacetonitriles with imines has been developed. Good yields and diastereo- and enantioselectivities were observed for the ... |
| di-tert-butylazodicarboxylate |
| EINECS 212-796-9 |
| MFCD00015001 |
| Bis(2-methyl-2-propanyl) 1,2-diazenedicarboxylate |
| Boc-N=N-Boc |
| Di-tert-Butyl azodic |
| tert-butyl azodicarboxylate |
| di-tert-butylazadicarboxylate |
| Di-tert-butyl azodicarboxylate |
| DI-TERT-BUTYL AZODICARBOXYALTE |
| DI-T-BUTYL AZODICARBOXYLATE |
| tBuO2CN=NCO2tBu |
| 1,2-Diazenedicarboxylic acid, bis(1,1-dimethylethyl) ester |
| Di-tert-butyl diazene-1,2-dicarboxylate |

 CAS#:345310-98-7
CAS#:345310-98-7 CAS#:185456-26-2
CAS#:185456-26-2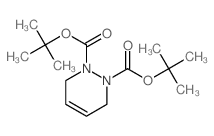 CAS#:13051-19-9
CAS#:13051-19-9![2,3-Diazabicyclo[2.2.1]heptane-2,3-dicarboxylicacid, 2,3-bis(1,1-dimethylethyl) ester structure](https://image.chemsrc.com/caspic/113/13051-18-8.png) CAS#:13051-18-8
CAS#:13051-18-8![tert-butyl N-[(2-methylpropan-2-yl)oxycarbonylamino]-N-phenylcarbamate structure](https://image.chemsrc.com/caspic/123/65578-58-7.png) CAS#:65578-58-7
CAS#:65578-58-7 CAS#:65813-90-3
CAS#:65813-90-3
