Potassium citrate
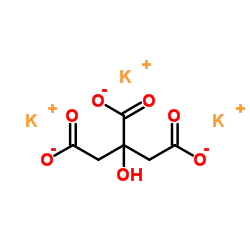
Potassium citrate structure
|
Common Name | Potassium citrate | ||
|---|---|---|---|---|
| CAS Number | 866-84-2 | Molecular Weight | 306.395 | |
| Density | 1.187 | Boiling Point | 309.6ºC at 760 mmHg | |
| Molecular Formula | C6H5K3O7 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 155.2ºC | |
| Name | Potassium citrate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.187 |
|---|---|
| Boiling Point | 309.6ºC at 760 mmHg |
| Molecular Formula | C6H5K3O7 |
| Molecular Weight | 306.395 |
| Flash Point | 155.2ºC |
| Exact Mass | 305.894653 |
| PSA | 140.62000 |
| Water Solubility | H2O: 1 M at 20 °C, clear, colorless |
Synonym:Potassium citric acid, tripotassium sal Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Not available. Potential Health Effects Eye: Dust may cause mechanical irritation. Skin: May cause mild skin irritation. Ingestion: Ingestion of large amounts may cause gastrointestinal irritation. Inhalation: Excessive inhalation may cause minor respiratory irritation. Chronic: No information found. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Extinguishing Media: For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Section 7 - HANDLING and STORAGE Handling: Use with adequate ventilation. Minimize dust generation and accumulation. Avoid prolonged or repeated contact with skin. Storage: Keep container closed when not in use. No special precautions indicated. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 866-84-2: Personal Protective Equipment Eyes: Wear safety glasses with side shields. Skin: Wear appropriate gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to minimize contact with skin. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: white Odor: Not available. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 230 deg C Autoignition Temperature: Not available. Flash Point: Not available. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Not available. Solubility in water: Very soluble in water Specific Gravity/Density: Not available. Molecular Formula: K3C6H5O7H2O Molecular Weight: 324.3391 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable. Conditions to Avoid: Not available. Incompatibilities with Other Materials: No data reported. Hazardous Decomposition Products: Oxides of phosphorus. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 866-84-2 unlisted. LD50/LC50: Not available. Carcinogenicity: Potassium citrate - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling. Section 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: WGK (Water Danger/Protection) CAS# 866-84-2: 0 Canada CAS# 866-84-2 is listed on Canada's DSL List. CAS# 866-84-2 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 866-84-2 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| HS Code | 2918150000 |
| HS Code | 2918150000 |
|---|---|
| Summary | 2918150000. other salts and esters of citric acid. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:4ABXY(export license,certificate of inspection for goods inward,certificate of inspection for goods outwardexport license(processing trade),export license(border small volume trade)). MFN tariff:6.5%. General tariff:30.0% |
|
The Characteristics of the Stone and Urine Composition in Chinese Stone Formers: Primary Report of a Single-center Results
Urology 83(4) , 732-7, (2014) Objective To assess urine composition in Chinese patients with urolithiasis. |
|
|
Nerve-targeted desensitizing toothpastes occlude dentin tubules and induce mineral precipitation.
Am. J. Dent. 25(1) , 26-30, (2012) To examine the laboratory dentin tubules occlusion and mineral precipitation capability of two potassium salts-containing desensitizing toothpastes.40 dentin disks were obtained and divided into four ... |
|
|
The effect of sodium bicarbonate upon urinary citrate excretion in calcium stone formers.
Urology 82(1) , 33-7, (2013) To evaluate the effects of oral sodium bicarbonate (NaBic) supplementation upon urinary citrate excretion in calcium stone formers (CSFs).Sixteen adult calcium stone formers with hypocitraturia were e... |
| Citric acid, tripotassium salt |
| EINECS 212-755-5 |
| SODIUM POTASSIUM |
| MFCD00036362 |
| kajos |
| 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, potassium salt (1:3) |
| tripotassium 2-hydroxypropane-1,2,3-tricarboxylate |
| Tripotassium citrate |
| seltz-k |
| UNII-86R1NVR0HW |
| sdTripotassium Citrate |
| citric acid, potassium salt |
| potassiumcitrateanhydrous |
| kaliksir |
| HYDROXY CITRIC ACID SYNTHETIC |
| porekal |
