20-HETE
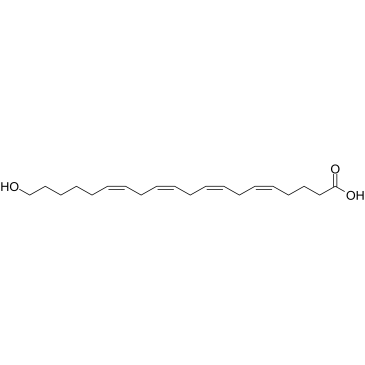
20-HETE structure
|
Common Name | 20-HETE | ||
|---|---|---|---|---|
| CAS Number | 79551-86-3 | Molecular Weight | 320.47 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 482.0±33.0 °C at 760 mmHg | |
| Molecular Formula | C20H32O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 259.4±21.9 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of 20-HETE20-HETE(20-hydroxy Arachidonic Acid) is a potent vasoconstrictor produced in vascular smooth muscle (VSM) cells. It depolarizes VSM by blocking the open-state probability of Ca2+-activated K+-channels.IC50 Value:Target: 20-Hydroxyeicosatetraenoic acid (20-HETE) is a cytochrome P450-derived arachidonic acid metabolite that has been shown to increase smooth muscle contractions and proliferation, stimulate endothelial dysfunction and activation and promote hypertension. in vitro: Addition of 20-HETE to the bath (1-100 nM), reduced the frequency of opening of the large-conductance Ca(2+)-activated K+ channel recorded using cell-attached patches on VSM [1]. In kidney, 20-HETE induces diuresis by inhibiting Na+-K+-ATPase in proximal tubules and Na+/K+/Cl+ cotransporter in the thick ascending limb of Henle's loop [2].in vivo: In Cyp4a14(-/-) mice, which display androgen-driven and 20-HETE-dependent hypertension, treatment with20-HETE antagonist abolished remodeling of renal resistance arteries measured as media thickness (24±1 vs. 15±1μm) and M/L (0.29±0.03 vs. 0.17±0.01) [4]. The transgenic mice had overexpressed hepatic CYP4F2, high hepatic 20-HETE and fasting plasma glucose levels but normal insulin level. The GP activity was increased and the cAMP/PKA-PhK-GP pathway was activated in the transgenic mice compared with wild-type mice [5]. Clinical trial: Mechanisms of Response to Diesel Exhaust in Subjects With Asthma. Phase not specified |
| Name | 20-hete |
|---|---|
| Synonym | More Synonyms |
| Description | 20-HETE(20-hydroxy Arachidonic Acid) is a potent vasoconstrictor produced in vascular smooth muscle (VSM) cells. It depolarizes VSM by blocking the open-state probability of Ca2+-activated K+-channels.IC50 Value:Target: 20-Hydroxyeicosatetraenoic acid (20-HETE) is a cytochrome P450-derived arachidonic acid metabolite that has been shown to increase smooth muscle contractions and proliferation, stimulate endothelial dysfunction and activation and promote hypertension. in vitro: Addition of 20-HETE to the bath (1-100 nM), reduced the frequency of opening of the large-conductance Ca(2+)-activated K+ channel recorded using cell-attached patches on VSM [1]. In kidney, 20-HETE induces diuresis by inhibiting Na+-K+-ATPase in proximal tubules and Na+/K+/Cl+ cotransporter in the thick ascending limb of Henle's loop [2].in vivo: In Cyp4a14(-/-) mice, which display androgen-driven and 20-HETE-dependent hypertension, treatment with20-HETE antagonist abolished remodeling of renal resistance arteries measured as media thickness (24±1 vs. 15±1μm) and M/L (0.29±0.03 vs. 0.17±0.01) [4]. The transgenic mice had overexpressed hepatic CYP4F2, high hepatic 20-HETE and fasting plasma glucose levels but normal insulin level. The GP activity was increased and the cAMP/PKA-PhK-GP pathway was activated in the transgenic mice compared with wild-type mice [5]. Clinical trial: Mechanisms of Response to Diesel Exhaust in Subjects With Asthma. Phase not specified |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 482.0±33.0 °C at 760 mmHg |
| Molecular Formula | C20H32O3 |
| Molecular Weight | 320.47 |
| Flash Point | 259.4±21.9 °C |
| Exact Mass | 320.235138 |
| PSA | 57.53000 |
| LogP | 4.91 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.515 |
| Storage condition | −20°C |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H319 |
| Precautionary Statements | P210-P305 + P351 + P338-P370 + P378-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | F: Flammable;Xi: Irritant; |
| Risk Phrases | 11-36/37/38 |
| Safety Phrases | 16-26-36 |
| RIDADR | UN 1170 3/PG 2 |
| WGK Germany | 3 |
|
HMDB: a knowledgebase for the human metabolome.
Nucleic Acids Res. 37(Database issue) , D603-10, (2009) The Human Metabolome Database (HMDB, http://www.hmdb.ca) is a richly annotated resource that is designed to address the broad needs of biochemists, clinical chemists, physicians, medical geneticists, ... |
|
|
The human serum metabolome.
PLoS ONE 6(2) , e16957, (2011) Continuing improvements in analytical technology along with an increased interest in performing comprehensive, quantitative metabolic profiling, is leading to increased interest pressures within the m... |
|
|
IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice.
J. Cereb. Blood Flow Metab. 34(12) , 1887-97, (2014) Aging impairs autoregulatory protection in the brain, exacerbating hypertension-induced cerebromicrovascular injury, neuroinflammation, and development of vascular cognitive impairment. Despite the im... |
| 20-hydroxy-5,8,11,14-eicosatetraenoic acid |
| 20-HETE |
| 20-Hydroxyicosatetraenoic acid |
| 5,8,11,14-Eicosatetraenoic acid, 20-hydroxy-, (5Z,8Z,11Z,14Z)- |
| DSR-II-247-30 |
| (5Z,8Z,11Z,14Z)-20-hydroxyicosa-5,8,11,14-tetraenoic acid |
| 20-Hydroxyeicosatetraenoic acid |
| 20-Hydroxyicosatetraenoate |
| 20-Hydroxy arachidonic acid |
| (5Z,8Z,11Z,14Z)-20-Hydroxy-5,8,11,14-icosatetraenoic acid |
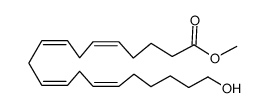 CAS#:86179-96-6
CAS#:86179-96-6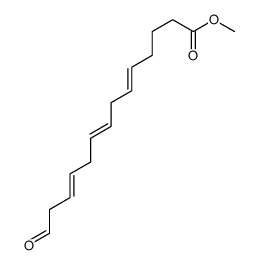 CAS#:86255-54-1
CAS#:86255-54-1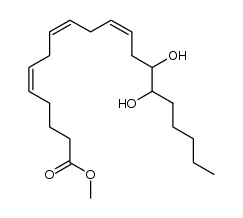 CAS#:195612-60-3
CAS#:195612-60-3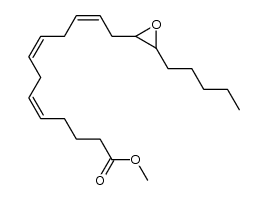 CAS#:197508-63-7
CAS#:197508-63-7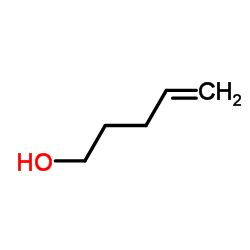 CAS#:821-09-0
CAS#:821-09-0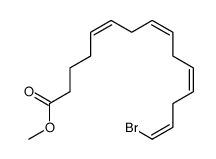 CAS#:688034-83-5
CAS#:688034-83-5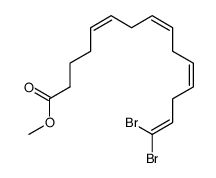 CAS#:688034-82-4
CAS#:688034-82-4