3-Indoleacetonitrile
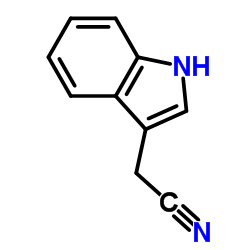
3-Indoleacetonitrile structure
|
Common Name | 3-Indoleacetonitrile | ||
|---|---|---|---|---|
| CAS Number | 771-51-7 | Molecular Weight | 156.184 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 374.1±17.0 °C at 760 mmHg | |
| Molecular Formula | C10H8N2 | Melting Point | 33-36 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 127.6±6.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3-Indoleacetonitrile3-Indoleacetonitrile is an endogenous metabolite. |
| Name | indole-3-acetonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Indoleacetonitrile is an endogenous metabolite. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 374.1±17.0 °C at 760 mmHg |
| Melting Point | 33-36 °C(lit.) |
| Molecular Formula | C10H8N2 |
| Molecular Weight | 156.184 |
| Flash Point | 127.6±6.1 °C |
| Exact Mass | 156.068741 |
| PSA | 39.58000 |
| LogP | 1.37 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.673 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36/37 |
| RIDADR | 3276 |
| WGK Germany | 3 |
| RTECS | AM0700000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 29339990 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Systematic profiling of indole-3-acetic acid biosynthesis in bacteria using LC-MS/MS.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 988 , 53-8, (2015) Indole-3-acetic acid (IAA) is produced from tryptophan through five synthesis pathways. A comprehensive method for the quantification of IAA and biosynthesis-related intermediates in a culture medium ... |
|
|
The extended version of restriction analysis approach for the examination of the ability of low-molecular-weight compounds to modify DNA in a cell-free system.
Food Chem. Toxicol. 75 , 118-27, (2015) One of the primary requirements in toxicology is the assessment of ability of chemicals to induce DNA covalent modification. There are several well-established methods used for this purpose such as (3... |
|
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators.
J. Med. Chem. 54 , 5320, (2011) Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance. IDO inhibition is thus an active... |
| Indole-3-acetonitrile |
| 3-Indolacetonitrile |
| (3-Indolyl)acetonitrile |
| (indol-3-yl)acetonitrile |
| 3-cyanomethyl-1H-indole |
| Indolylacetonitrile |
| MFCD00005628 |
| 3-Indolylacetonitrile |
| Indolylacetonitril |
| Indole-3-acetonitrile (8CI) |
| usafcb-29 |
| 3-acetonitrilindole |
| EINECS 212-232-1 |
| 1H-Indol-3-ylacetonitrile |
| 3-indolyl acetonitrile |
| (1H-Indol-3-yl)acetonitrile |
| 3-Indoleacetonitrile |
| IAN |
| 3-indolyl-acetonitril |
| 1H-Indole-3-acetonitrile |
| Indoleacetonitrile |
| 3-ICN |
| 3-cyanomethylindole |
 CAS#:87-52-5
CAS#:87-52-5 CAS#:143-33-9
CAS#:143-33-9 CAS#:115610-85-0
CAS#:115610-85-0 CAS#:109662-79-5
CAS#:109662-79-5 CAS#:60438-65-5
CAS#:60438-65-5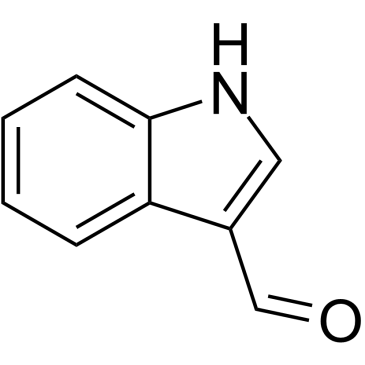 CAS#:487-89-8
CAS#:487-89-8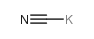 CAS#:151-50-8
CAS#:151-50-8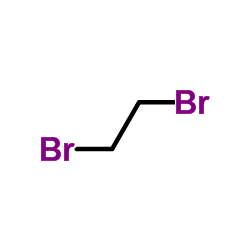 CAS#:106-93-4
CAS#:106-93-4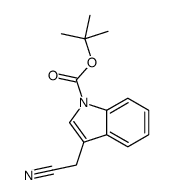 CAS#:218772-62-4
CAS#:218772-62-4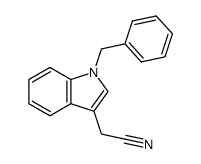 CAS#:59414-85-6
CAS#:59414-85-6 CAS#:106050-92-4
CAS#:106050-92-4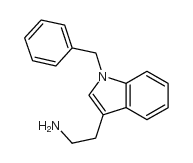 CAS#:4307-98-6
CAS#:4307-98-6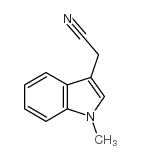 CAS#:51584-17-9
CAS#:51584-17-9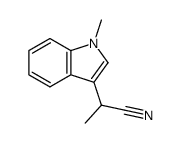 CAS#:176688-64-5
CAS#:176688-64-5 CAS#:19853-01-1
CAS#:19853-01-1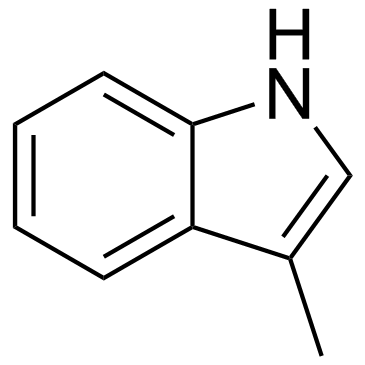 CAS#:83-34-1
CAS#:83-34-1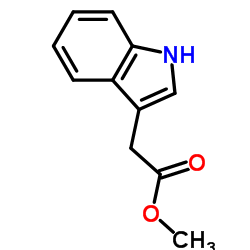 CAS#:1912-33-0
CAS#:1912-33-0 CAS#:879-37-8
CAS#:879-37-8
