Myxothiazol
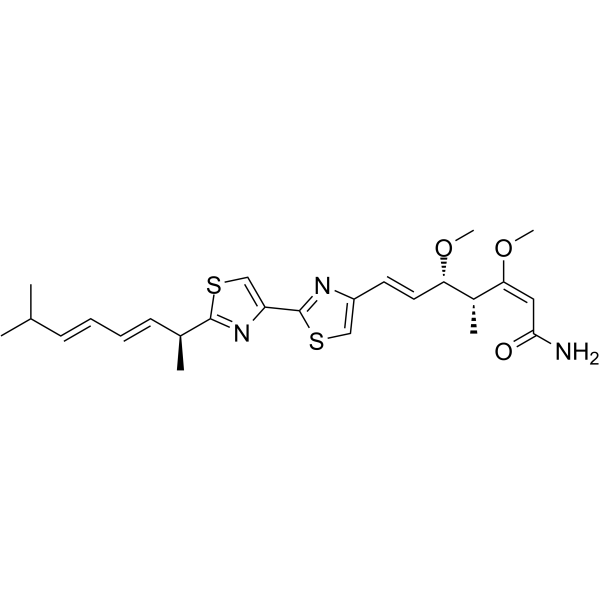
Myxothiazol structure
|
Common Name | Myxothiazol | ||
|---|---|---|---|---|
| CAS Number | 76706-55-3 | Molecular Weight | 487.67800 | |
| Density | 1.158g/cm3 | Boiling Point | 679.6ºC at 760 mmHg | |
| Molecular Formula | C25H33N3O3S2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 364.8ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of MyxothiazolMyxothiazol, an antifungal antibiotic, is a mitochondrial electron transport chain complex III (bc1 complex) inhibitor. Myxothiazol inhibits the growth of many yeasts and fungi at concentrations between 0.01 and 3 μg/ml[1][2]. |
| Name | myxothiazol |
|---|---|
| Synonym | More Synonyms |
| Description | Myxothiazol, an antifungal antibiotic, is a mitochondrial electron transport chain complex III (bc1 complex) inhibitor. Myxothiazol inhibits the growth of many yeasts and fungi at concentrations between 0.01 and 3 μg/ml[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Myxothiazol inhibits the growth of many yeasts and fungi at concentrations between 0.01 and 3 μg/ml[2]. Myxothiazol binds to the ubiquinol oxidation site Qo of complex III and blocks electron transfer from ubiquinol to cytochrome b and thus inhibits complex III activity[3]. |
| In Vivo | Myxothiazol (i.p.; 0.56 mg/kg; daily for 4 days)-induced complex III inhibition can be induced in mice for four days in a row without overt hepatotoxicity or lethality[3]. Animal Model: C57Bl/J6 mice[3] Dosage: 0.56 mg/kg Administration: I.p.; 24 hours intervals for at most 4 times Result: A reversible complex III activity decrease to 50% of control value occurred at 2 h post-injection. At 74 h only minor histological changes in the liver were found, supercomplex formation was preserved and no significant changes in the expression of genes indicating hepatotoxicity or inflammation were found. |
| References |
| Density | 1.158g/cm3 |
|---|---|
| Boiling Point | 679.6ºC at 760 mmHg |
| Molecular Formula | C25H33N3O3S2 |
| Molecular Weight | 487.67800 |
| Flash Point | 364.8ºC |
| Exact Mass | 487.19600 |
| PSA | 143.81000 |
| LogP | 6.51870 |
| Index of Refraction | 1.584 |
| InChIKey | XKTFQMCPGMTBMD-FYHMSGCOSA-N |
| SMILES | COC(=CC(N)=O)C(C)C(C=Cc1csc(-c2csc(C(C)C=CC=CC(C)C)n2)n1)OC |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Hazard Codes | T+ |
| Risk Phrases | 28 |
| Safety Phrases | 28-36/37-45 |
| RIDADR | UN 3462 6.1/PG 1 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~59% 
Myxothiazol CAS#:76706-55-3 |
| Literature: Iwaki, Yuki; Kaneko, Masahiro; Akita, Hiroyuki Tetrahedron Asymmetry, 2009 , vol. 20, # 3 p. 298 - 304 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Unsuspected pyocyanin effect in yeast under anaerobiosis.
Microbiologyopen 3(1) , 1-14, (2014) The blue-green phenazine, Pyocyanin (PYO), is a well-known virulence factor produced by Pseudomonas aeruginosa, notably during cystic fibrosis lung infections. It is toxic to both eukaryotic and bacte... |
|
|
Oxidized LDL-induced angiogenesis involves sphingosine 1-phosphate: prevention by anti-S1P antibody.
Br. J. Pharmacol. 172(1) , 106-18, (2014) Neovascularization occurring in atherosclerotic lesions may promote plaque expansion, intraplaque haemorrhage and rupture. Oxidized LDL (oxLDL) are atherogenic, but their angiogenic effect is controve... |
|
|
The small molecule C-6 is selectively cytotoxic against breast cancer cells and its biological action is characterized by mitochondrial defects and endoplasmic reticulum stress.
Breast Cancer Res. 16(6) , 472, (2015) The establishment of drug resistance following treatment with chemotherapeutics is strongly associated with poor clinical outcome in patients, and drugs that target chemoresistant tumors have the pote... |
| 2,6-Heptadienamide,7-(2'-((1S,2E,4E)-1,6-dimethyl-2,4-heptadienyl)(2,4'-bithiazol)-4-yl)-3,5-dimethoxy-4-methyl-,(2E,4R,5S,6E) |
| MFCD00043397 |
| MYXOTHIAZOL |
| 7-(2 |
| 5-dimethoxy-4-methyl |
| 2,6-Heptadienamide,7-(2'-(1,6-dimethyl-2,4-heptadienyl)(2,4'-bithiazol)-4-yl)-3,5-dimethoxy-4-methyl |
![4-(2''-benzothiazolyl)sulfonylmethyl-2'-[(1'''S),6'''-dimethylhepta-(2'''E),(4'''E)-dienyl]-2,4'-bithiazole structure](https://image.chemsrc.com/caspic/398/1093126-36-3.png)