(R)-(-)-α-Methylhistamine dihydrochloride
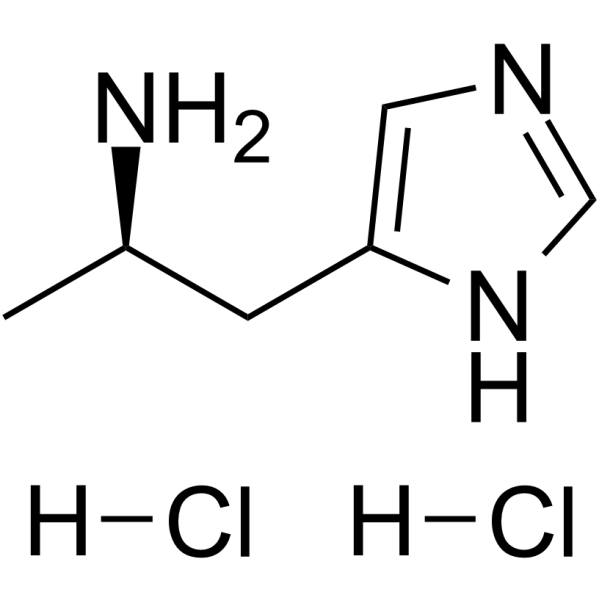
(R)-(-)-α-Methylhistamine dihydrochloride structure
|
Common Name | (R)-(-)-α-Methylhistamine dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 75614-89-0 | Molecular Weight | 198.09400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H13Cl2N3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (R)-(-)-α-Methylhistamine dihydrochloride(R)-(-)-α-Methylhistamine dihydrochloride is a potent, selective and brain-penetrant agonist of H3 histamine receptor, with a Kd of 50.3 nM[1][2]. (R)-(-)-α-Methylhistamine dihydrochloride can enhance memory retention, attenuates memory impairment in rats[3][4][5]. |
| Name | R(-)-α-Methyl Histamine Dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-(-)-α-Methylhistamine dihydrochloride is a potent, selective and brain-penetrant agonist of H3 histamine receptor, with a Kd of 50.3 nM[1][2]. (R)-(-)-α-Methylhistamine dihydrochloride can enhance memory retention, attenuates memory impairment in rats[3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
H3 Receptor:50.3 nM (Kd) |
| In Vitro | (R)-(-)-α-Methylhistamine dihydrochloride is an H3-agonist that is >10 times as potent as histamine (HA). Its selectivity toward H3-receptors is >1000 times as high as that of HA. (R)-(-)-α-Methylhistamine dihydrochloride has only weak affinities for H1 and H2 receptor with a pKi=4.8 and <3.5, repectively. (R)-(-)-α-Methylhistamine dihydrochloride displays >200-fold selectivity over H4 receptors[1][2][3]. |
| In Vivo | Pretreatment with (R)-(-)-α-Methylhistamine dihydrochloride (RAMH; 10 mg/kg; i.p.; 60 min before training) reverses Propofol‐induced (25 mg/kg; i.p.; 30 min before training) memory retention[5]. (R)-(-)-α-Methylhistamine dihydrochloride (6.3 mg/kg; i.p.) significantly decreases the steady-state t-MH level in the mouse brain, whereas these compounds produced no significant changes in the HA level[3]. Animal Model: Male Sprague‐Dawley rats (10-12 week)[3] Dosage: 10 mg/kg Administration: IP; 60 min before training Result: Reversed propofol‐induced memory retention. |
| References |
| Molecular Formula | C6H13Cl2N3 |
|---|---|
| Molecular Weight | 198.09400 |
| Exact Mass | 197.04900 |
| PSA | 54.70000 |
| LogP | 2.77620 |
| InChIKey | IZHCNQFUWDFPCW-ZJIMSODOSA-N |
| SMILES | CC(N)Cc1cnc[nH]1.Cl.Cl |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
|
Highly potent and selective ligands for histamine H3-receptors.
Nature 327 , 117, (1987) New drugs selective for histamine H3-receptors can be used to establish that these receptors are involved in the feedback control of histamine synthesis and release, and to demonstrate their distribut... |
|
|
Effects of the histamine H3-agonist (R)-alpha-methylhistamine and the antagonist thioperamide on histamine metabolism in the mouse and rat brain.
J. Neurochem. 52 , 1388, (1989) To study the feedback control by histamine (HA) H3-receptors on the synthesis and release of HA at nerve endings in the brain, the effects of a potent and selective H3-agonist, (R)-alpha-methylhistami... |
|
|
Structure-activity relations of histamine analogs. XX. Absolute configuration and histamine-like activity of enantiomeric α-methyl histamines. Gerhard, et al.
Arch. Pharm. Res. 313 , 709, (1980)
|
| R(-)-α-Methylhistamine Dihydrochloride |