Moxonidine hydrochloride
Modify Date: 2025-08-25 18:32:24
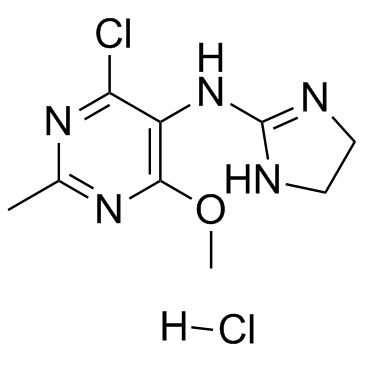
Moxonidine hydrochloride structure
|
Common Name | Moxonidine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 75536-04-8 | Molecular Weight | 278.138 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C9H13Cl2N5O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Moxonidine hydrochlorideMoxonidine Hydrochloride is a selective agonist at the imidazoline receptor subtype 1, used as antihypertensive agent.Target: I1-RMoxonidine Hydrochloride is a centrally acting antihypertensive agent. Mixed Nischarin (I1 imidazoline receptor) and α2-AR (adrenergic) agonist; displays 40-fold higher affinity for I1 receptors versus α2-adrenoceptors. Moxonidine reduced stimulated NE overflow (log EC50: -6.15 +/- 0.14). AGN192403, a selective ligand at I1-R, had no influence on the dose-response curve of moxonidine (log EC50: -6.01 +/- 0.25) [1]. The hypotensive and bradycardic actions of moxonidine but not clonidine are mediated through imidazoline receptors and are dependent on intact noradrenergic pathways within the RVLM. Furthermore, the noradrenergic innervation may be associated with a 42 kDa imidazoline receptor protein [2]. |
| Name | Moxonidine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Moxonidine Hydrochloride is a selective agonist at the imidazoline receptor subtype 1, used as antihypertensive agent.Target: I1-RMoxonidine Hydrochloride is a centrally acting antihypertensive agent. Mixed Nischarin (I1 imidazoline receptor) and α2-AR (adrenergic) agonist; displays 40-fold higher affinity for I1 receptors versus α2-adrenoceptors. Moxonidine reduced stimulated NE overflow (log EC50: -6.15 +/- 0.14). AGN192403, a selective ligand at I1-R, had no influence on the dose-response curve of moxonidine (log EC50: -6.01 +/- 0.25) [1]. The hypotensive and bradycardic actions of moxonidine but not clonidine are mediated through imidazoline receptors and are dependent on intact noradrenergic pathways within the RVLM. Furthermore, the noradrenergic innervation may be associated with a 42 kDa imidazoline receptor protein [2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C9H13Cl2N5O |
|---|---|
| Molecular Weight | 278.138 |
| Exact Mass | 277.049713 |
| Storage condition | 2-8℃ |
| 5-Pyrimidinamine, 4-chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxy-2-methyl-, hydrochloride (1:1) |
| 4-Chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxy-2-methylpyrimidin-5-amine hydrochloride (1:1) |
| 5RYW07SK5E |
| Moxonidine hydrochloride |
| Moxonidine HCl |
| 4-Chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxy-2-methyl-5-pyrimidinamine hydrochloride (1:1) |
| MFCD06795643 |