Zalcitabine
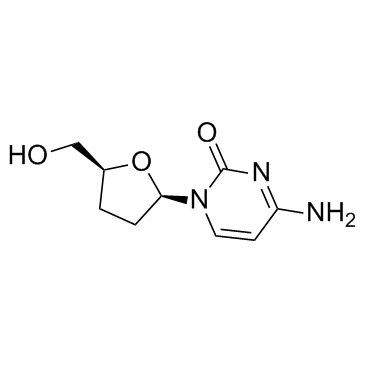
Zalcitabine structure
|
Common Name | Zalcitabine | ||
|---|---|---|---|---|
| CAS Number | 7481-89-2 | Molecular Weight | 211.218 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 415.0±55.0 °C at 760 mmHg | |
| Molecular Formula | C9H13N3O3 | Melting Point | 217-218 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 204.8±31.5 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of ZalcitabineZalcitabine is a potent nucleoside analogue reverse transcriptase inhibitor used in the treatment of HIV infection. |
| Name | zalcitabine |
|---|---|
| Synonym | More Synonyms |
| Description | Zalcitabine is a potent nucleoside analogue reverse transcriptase inhibitor used in the treatment of HIV infection. |
|---|---|
| Related Catalog | |
| Target |
Target: HIV |
| In Vitro | Zalcitabine is a dideoxynucleoside antiretroviral agent that is phosphorylated to the active metabolite 2',3'-dideoxycytidine 5'-triphosphate (ddCTP) within both uninfected and HIV-infected cells. At therapeutic concentrations, ddCTP inhibits HIV replication by inhibiting the enzyme reverse transcriptase and terminating elongation of the proviral DNA chain[1]. Zalcitabine exhibits the inhibition effect on the cellular uptake of [3H]-PAH in CHO/hOAT1 cells with an IC50 value of 1.23 mM. Furthermore, the cellular uptake of zalcitabine increased threefold with the enhancement of hOATI activity in CHO/hOAT1 cells[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 415.0±55.0 °C at 760 mmHg |
| Melting Point | 217-218 °C(lit.) |
| Molecular Formula | C9H13N3O3 |
| Molecular Weight | 211.218 |
| Flash Point | 204.8±31.5 °C |
| Exact Mass | 211.095688 |
| PSA | 90.37000 |
| LogP | -1.30 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.686 |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | 5-10 g/100 mL at 19 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R40 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HA3870000 |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Absence of a universal mechanism of mitochondrial toxicity by nucleoside analogs.
Antimicrob. Agents Chemother. 51 , 2531-9, (2007) Nucleoside analogs are associated with various mitochondrial toxicities, and it is becoming increasingly difficult to accommodate these differences solely in the context of DNA polymerase gamma inhibi... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| ddCyd |
| [3H]-Zalcitabine |
| 2',3'-DIDEOXYCYTIDINE |
| 4-Amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one |
| DDC |
| DDC (VAN) |
| Zalcitabine |
| 1-[(2R,5S)-5-(Hydroxymethyl)tetrahydro-2-furanyl]-4-imino-1,4-dihydro-2-pyrimidinol |
| 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one |
| HIVID |
| MFCD00012188 |
| 2-Pyrimidinol, 1,4-dihydro-4-imino-1-[(2R,5S)-tetrahydro-5-(hydroxymethyl)-2-furanyl]- |
| 4-Amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydro-2-furanyl]-2(1H)-pyrimidinone |
| Cytidine,2',3'-dideoxy |
| Dideoxycytidine |
| 3'-Azido-3'-deoxythymidine |
| 4-Amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-on |
| Zalcitibine |
| 2',3'-Dideoxycytidinene |
| CYTIDINE, 2',3'-DIDEOXY- |
| ddC (Antiviral) |
| 2(1H)-Pyrimidinone, 4-amino-1-[(2R,5S)-tetrahydro-5-(hydroxymethyl)-2-furanyl]- |
| 4-amino-1-[(2R,5S)-5-(hydroxyméthyl)tétrahydrofuran-2-yl]pyrimidin-2(1H)-one |
 CAS#:7481-88-1
CAS#:7481-88-1 CAS#:119818-52-9
CAS#:119818-52-9 CAS#:119794-42-2
CAS#:119794-42-2 CAS#:3768-18-1
CAS#:3768-18-1![1-[2,3-dideoxy-5-O-(4-phenybenzoyl)-β-D-glycero-pentofuranosyl]-4-(isobutyrylamino)-2(1H)-pyrimidinone*0.25H2O Structure](https://image.chemsrc.com/caspic/463/125612-00-2.png) CAS#:125612-00-2
CAS#:125612-00-2 CAS#:120885-66-7
CAS#:120885-66-7![N-[1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]benzamide Structure](https://image.chemsrc.com/caspic/433/120885-60-1.png) CAS#:120885-60-1
CAS#:120885-60-1 CAS#:5983-09-5
CAS#:5983-09-5
