Cyheptamide
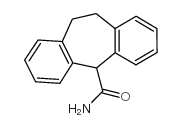
Cyheptamide structure
|
Common Name | Cyheptamide | ||
|---|---|---|---|---|
| CAS Number | 7199-29-3 | Molecular Weight | 237.29600 | |
| Density | 1.173 g/cm3 | Boiling Point | 438.8ºC at 760 mmHg | |
| Molecular Formula | C16H15NO | Melting Point | 193-194ºC | |
| MSDS | Chinese USA | Flash Point | 219.2ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CyheptamideCyheptamide is an anticonvulsant. |
| Name | 6,11-dihydro-5H-dibenzo[1,2-a:1',2'-e][7]annulene-11-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.173 g/cm3 |
|---|---|
| Boiling Point | 438.8ºC at 760 mmHg |
| Melting Point | 193-194ºC |
| Molecular Formula | C16H15NO |
| Molecular Weight | 237.29600 |
| Flash Point | 219.2ºC |
| Exact Mass | 237.11500 |
| PSA | 43.09000 |
| LogP | 3.10270 |
| Index of Refraction | 1.62 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| RIDADR | NONH for all modes of transport |
| RTECS | HO7878000 |
| HS Code | 2924299090 |
|
~% 
Cyheptamide CAS#:7199-29-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 251 - 255 |
|
~% 
Cyheptamide CAS#:7199-29-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 251 - 255 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
A simple, efficient, and sensitive method for simultaneous detection of anti-HIV drugs atazanavir, ritonavir, and tenofovir by use of liquid chromatography-tandem mass spectrometry.
Antimicrob. Agents Chemother. 59 , 6682-8, (2015) In the treatment of HIV infection, a combination of anti-HIV drugs is commonly used in highly active antiretroviral therapy (HAART). One such combination recommended for clinical therapy consists of t... |
|
|
Evaluation of atazanavir and darunavir interactions with lipids for developing pH-responsive anti-HIV drug combination nanoparticles.
J. Pharm. Sci. 103(8) , 2520-9, (2014) We evaluated two human immunodeficiency virus (HIV) protease inhibitors, atazanavir (ATV) and darunavir (DRV), for pH-dependent solubility, lipid binding, and drug release from lipid nanoparticles (LN... |
|
|
Cyheptamide and 3-hydroxy-3-phenacyloxindole: structural similarity to diphenylhydantoin as the basis for anticonvulsant activity.
J. Med. Chem. 27(5) , 649-54, (1984) The molecular structures of cyheptamide and 3-hydroxy-3- phenacyloxindole were determined by X-ray diffraction methods. The amide group in both compounds exhibits delocalization of the pi-electrons ov... |
| Cyheptamine |
| BS 7029 |
| AY 8682 |
| EINECS 230-570-8 |
| CYHEPTAMIDE |
| Dibenzo<a,d>1,4-cycloheptadien-5-carboxamid |
| 5-Carbamoyl-10,11-dihydro-5H-dibenzo<a,d>cyclohepten |
![10,11-Dihydro-5H-dibenzo[a,d]cycloheptene-5-carbonitrile structure](https://image.chemsrc.com/caspic/457/1729-63-1.png)

