Cefpiramide acid
Modify Date: 2025-08-25 09:29:08
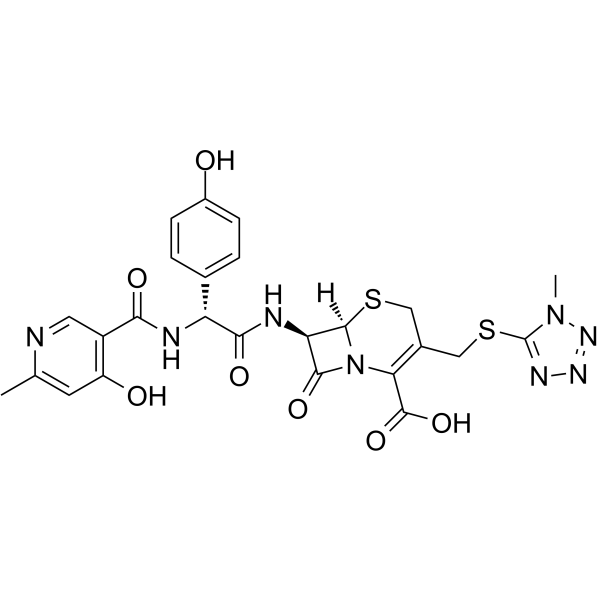
Cefpiramide acid structure
|
Common Name | Cefpiramide acid | ||
|---|---|---|---|---|
| CAS Number | 70797-11-4 | Molecular Weight | 612.63700 | |
| Density | 1.75 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C25H24N8O7S2 | Melting Point | 213-215° (dec) | |
| MSDS | N/A | Flash Point | N/A | |
Use of Cefpiramide acidCefpiramide (SM-1652) free acid is a semisynthetic cephalosporin with broad-spectrum antibacterial activity. Cefpiramide free acid shows strong antibacterial effect on both gram-positive bacteria and gram-negative bacteria. Cefpiramide free acid is moderately susceptible to β-lactamase[1][2]. |
| Name | cefpiramide |
|---|---|
| Synonym | More Synonyms |
| Description | Cefpiramide (SM-1652) free acid is a semisynthetic cephalosporin with broad-spectrum antibacterial activity. Cefpiramide free acid shows strong antibacterial effect on both gram-positive bacteria and gram-negative bacteria. Cefpiramide free acid is moderately susceptible to β-lactamase[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Bacterial[1][2] |
| In Vitro | Cefpiramide (0-2048 µg/mL; 16-18 hours) shows good activity to against most non-fermentative Gram-negative bacilli and Enterococci (32 µg/mL with 97% inhibition)[1]. Cefpiramide (0-2048 µg/mL; 16-18 hours) exhibits high effective to against Pseudomonas aeruginosa with MIC[50] and MIC[90] values of 4 µg/mL and 16 µg/mL separately[1]. Cell Viability Assay[1] Cell Line: 761 bacterial isolates (35 different species of common bacterial pathogens) Concentration: 0-2048 µg/mL Incubation Time: 16-18 hours Result: Showed broad-spectrum antibacterial activity. |
| In Vivo | Cefpiramide (25 mg/kg; i.v.; once) shows anti-Streptococcus pneumoniae activity in experimental pneumococcal meningitis[2]. Cefpiramide (25 mg/kg; i.v.; once) reduces bacteria in CSF more than 10[4] CFU/ml[2]. Animal Model: New Zealand white male rabbits(2-3 kg)[2]. Dosage: 25 mg/kg Administration: Intravenous injection; once. Result: Anti-Streptococcus pneumoniae. |
| References |
| Density | 1.75 g/cm3 |
|---|---|
| Melting Point | 213-215° (dec) |
| Molecular Formula | C25H24N8O7S2 |
| Molecular Weight | 612.63700 |
| Exact Mass | 612.12100 |
| PSA | 263.36000 |
| LogP | 1.04640 |
| Index of Refraction | 1.821 |
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 3003201900 |
| HS Code | 3003201900 |
|---|
| CEFPIRAMIDE |
| Cefpiramide acid |