carbazeran
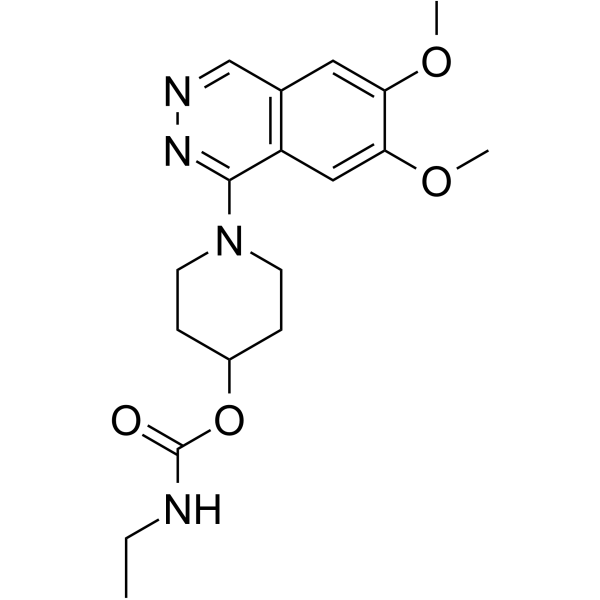
carbazeran structure
|
Common Name | carbazeran | ||
|---|---|---|---|---|
| CAS Number | 70724-25-3 | Molecular Weight | 360.408 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 595.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H24N4O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 314.0±30.1 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of carbazeranCarbazeran, a potent phosphodiesterase inhibitor, is aldehyde oxidase substrate. Carbazeran can be used for the research of metabolic disease[1]. |
| Name | 1-(6,7-Dimethoxyphthalazin-1-yl)piperidin-4-yl ethylcarbamate |
|---|---|
| Synonym | More Synonyms |
| Description | Carbazeran, a potent phosphodiesterase inhibitor, is aldehyde oxidase substrate. Carbazeran can be used for the research of metabolic disease[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 595.6±50.0 °C at 760 mmHg |
| Molecular Formula | C18H24N4O4 |
| Molecular Weight | 360.408 |
| Flash Point | 314.0±30.1 °C |
| Exact Mass | 360.179749 |
| PSA | 85.81000 |
| LogP | 1.38 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.600 |
| Storage condition | 2-8°C |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | T |
| RIDADR | UN 2811 6.1 / PGIII |
| HS Code | 2933990090 |
|
~% 
carbazeran CAS#:70724-25-3 |
| Literature: US4289772 A1, ; US 4289772 A |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Aldehyde oxidase activity in fresh human skin.
Drug Metab. Dispos. 42(12) , 2049-57, (2014) Human aldehyde oxidase (AO) is a molybdoflavoenzyme that commonly oxidizes azaheterocycles in therapeutic drugs. Although high metabolic clearance by AO resulted in several drug failures, existing in ... |
|
|
A novel reaction mediated by human aldehyde oxidase: amide hydrolysis of GDC-0834.
Drug Metab. Dispos. 43 , 908-15, (2015) GDC-0834, a Bruton's tyrosine kinase inhibitor investigated as a potential treatment of rheumatoid arthritis, was previously reported to be extensively metabolized by amide hydrolysis such that no mea... |
|
|
Chronotropic and inotropic actions of amrinone, carbazeran and isobutylmethyl xanthine: role of phosphodiesterase inhibition.
Br. J. Pharmacol. 98 , 291-301, (1999) 1. The chronotropic and inotropic effects of amrinone, carbazeran and 3-isobutyl-1-methyl xanthine (IBMX) were examined in isolated preparations of papillary muscle and right atria from rabbit heart. ... |
| Carbamic acid, ethyl-, 1-(6,7-dimethoxy-1-phthalazinyl)-4-piperidinyl ester |
| Carbamic acid, N-ethyl-, 1-(6,7-dimethoxy-1-phthalazinyl)-4-piperidinyl ester |
| 1-(6,7-Dimethoxyphthalazin-1-yl)piperidin-4-yl ethylcarbamate |
| [1-(6,7-dimethoxyphthalazin-1-yl)piperidin-4-yl] N-ethylcarbamate |
| 1-(6,7-Dimethoxy-1-phthalazinyl)-4-piperidinyl ethylcarbamate |
| carbazeran |
| Ethylcarbamic Acid 1-(6,7-Dimethoxy-1-phthalazinyl)-4-piperidinyl Ester |