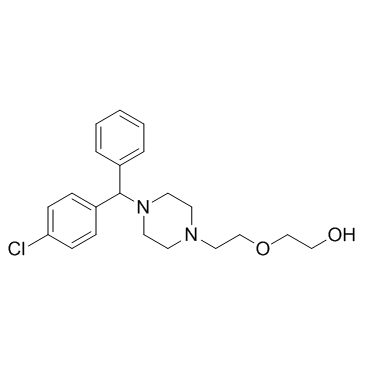
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KK2275000
-
CHEMICAL NAME :
-
Ethanol, 2-(2-(4-(p-chloro-alpha-phenylbenzyl)-1-piperazinyl)e thoxy)-
-
CAS REGISTRY NUMBER :
-
68-88-2
-
BEILSTEIN REFERENCE NO. :
-
0321392
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
11
-
MOLECULAR FORMULA :
-
C21-H27-Cl-N2-O2
-
MOLECULAR WEIGHT :
-
374.95
-
WISWESSER LINE NOTATION :
-
T6N DNTJ AYR&R D6& D2O2Q
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
840 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CHTPBA Chimica Therapeutica. (Paris, France) V.1-8, 1965-73. For publisher information, see EJMCA5. Volume(issue)/page/year: 3,210,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
160 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CHTPBA Chimica Therapeutica. (Paris, France) V.1-8, 1965-73. For publisher information, see EJMCA5. Volume(issue)/page/year: 3,210,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
45 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ANPBAZ Acta Neurologia et Psychiatrica Belgica. (Brussels, Belgium) V.48-69, 1948-69. For publisher information, see ANUBBR. Volume(issue)/page/year: 61,669,1961
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CHTPBA Chimica Therapeutica. (Paris, France) V.1-8, 1965-73. For publisher information, see EJMCA5. Volume(issue)/page/year: 3,210,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
81300 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DPHFAK Dissertationes Pharmaceuticae et Pharmacologicae. (Warsaw, Poland) V.18-24, 1966-72. For publisher information, see PJPPAA. Volume(issue)/page/year: 23,281,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
137 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZQAG "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972 Volume(issue)/page/year: -,237,1972 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 13-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - homeostasis
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 17,67,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
FESTAS Fertility and Sterility. (American Fertility Soc., 608 13th Ave. S, Birmingham, AL 35282) V.1- 1950- Volume(issue)/page/year: 12,346,1961
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
350 mg/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
28QFAD "Laboratory Animals in Drug Testing, Symposium of the 5th International Committee on Laboratory Animals, Hanover, 1972," Speigel, A. ed., Stuttgart, Fed. Rep. Ger., Gustav Fischer Verlag, 1973 Volume(issue)/page/year: -,233,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 1-60 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
REFERENCE :
-
28QFAD "Laboratory Animals in Drug Testing, Symposium of the 5th International Committee on Laboratory Animals, Hanover, 1972," Speigel, A. ed., Stuttgart, Fed. Rep. Ger., Gustav Fischer Verlag, 1973 Volume(issue)/page/year: -,233,1973 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X6151 No. of Facilities: 52 (estimated) No. of Industries: 1 No. of Occupations: 3 No. of Employees: 1362 (estimated) No. of Female Employees: 719 (estimated)
|