10246-75-0
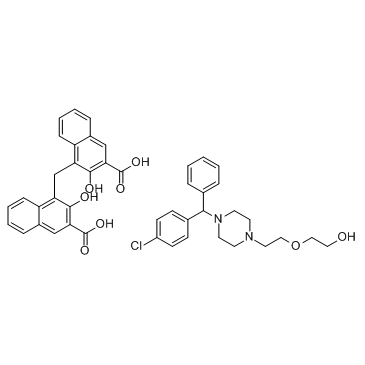
| Name | hydroxyzine pamoate |
|---|---|
| Synonyms |
Paxistil {Pamoate}
Equipose {Pamoate} 4,4'-Methylenebis(3-hydroxy-2-naphthoic acid) - 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]-1-piperazinyl}ethoxy)ethanol (1:1) Paxisitil 4,4'-methanediylbis(3-hydroxynaphthalene-2-carboxylic acid) - 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)ethanol (1:1) Bobsule Atarax P Hydroxyzine pamoate salt 4-[(3-carboxy-2-hydroxynaphthalen-1-yl)methyl]-3-hydroxynaphthalene-2-carboxylic acid,2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]ethanol hydroxyzine pamoate acide 4,4'-méthanediylbis(3-hydroxynaphtalène-2-carboxylique) - 2-(2-{4-[(4-chlorophényl)(phényl)méthyl]pipérazin-1-yl}éthoxy)éthanol (1:1) 2-Naphthalenecarboxylic acid, 4,4'-methylenebis[3-hydroxy-, compd. with 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]ethanol (1:1) Hydroxyzyne pamoate 4,4'-Methandiylbis(3-hydroxynaphthalen-2-carbonsäure)--2-(2-{4-[(4-chlorphenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)ethanol(1:1) EINECS 233-582-1 Hydroxyzine embonate Hydroxyzine (pamoate) |
| Description | Hydroxyzine pamoate is a histamine H1-receptor antagonist.Target: Histamine H1-ReceptorHydroxyzine inhibits carbachol (10 μM)-induced serotonin release by 34% at 10 μM, by 25% 1 μM and by 17% 0.1 μM in pretreated bladder slices for 60 min [1]. Hydroxyzine (0.1 mM) treatment inhibits the progression and severity of EAE by 50% and the extent of mast cell degranulation by 70% in Lewis rats with allergic encephalomyelitis (EAE) [2]. Hydroxyzine (500 M) significantly increases transport of etoposide to the serosal site in the jejunal everted sacs. Hydroxyzine significantly reduces the efflux and approximately 2.4 ug/mL of etoposide in the jejunum and ileum. Hydroxyzine (0.2 μg/mL) significantly enhances the efflux of RH123 to the lumen [3].Hydroxyzine (500 μM) significantly decreases the steady-state etoposide concentration 2-fold, where the steady-state concentration reached about 0.055 μM/mL in Sprague-Dawley rats [3]. Hydroxyzine (12.5 mg/kg, 25 mg/kg and 50 mg/kg i.p.) shows little direct analgesic activity but markedly potentiates only the effect of morphine on the vocalization after-discharge which represents the affective component of pain in rats. Hydroxyzine (50 mg/kg i.p.) potentiates morphine on the tail-flick test, while Hydroxyzine (12.5 mg/kg i.p.) decreases morphine antinociception in rats [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.182g/cm3 |
|---|---|
| Boiling Point | 499.2ºC at 760mmHg |
| Molecular Formula | C44H43ClN2O8 |
| Molecular Weight | 763.274 |
| Flash Point | 255.7ºC |
| Exact Mass | 762.270813 |
| PSA | 151.00000 |
| LogP | 7.32310 |
| Vapour Pressure | 2.13E-17mmHg at 25°C |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |