prochloraz
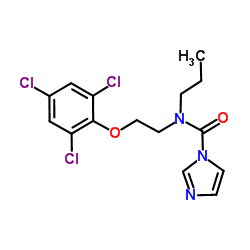
prochloraz structure
|
Common Name | prochloraz | ||
|---|---|---|---|---|
| CAS Number | 67747-09-5 | Molecular Weight | 376.665 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 499.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H16Cl3N3O2 | Melting Point | 46-49°C | |
| MSDS | Chinese USA | Flash Point | 256.1±31.5 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of prochlorazProchloraz is an imidazole antifungal that inhibits ergosterol biosynthesis via inhibition of the cytochrome P450-dependent 14α-demethylation of lanosterol, which results in disruption of the fungal cell membrane and cell death. Prochloraz inhibits human placenta microsomal aromatase in vitro (IC50 = 40 nM). Prochloraz also acts as an antagonist of the estrogen receptor (ER) and androgen receptor (AR) (IC50s = 25 μM and 4 μM, respectively) as well as activates the aryl hydrocarbon receptor (AhR; EC50 = 1 μM). |
| Name | prochloraz |
|---|---|
| Synonym | More Synonyms |
| Description | Prochloraz is an imidazole antifungal that inhibits ergosterol biosynthesis via inhibition of the cytochrome P450-dependent 14α-demethylation of lanosterol, which results in disruption of the fungal cell membrane and cell death. Prochloraz inhibits human placenta microsomal aromatase in vitro (IC50 = 40 nM). Prochloraz also acts as an antagonist of the estrogen receptor (ER) and androgen receptor (AR) (IC50s = 25 μM and 4 μM, respectively) as well as activates the aryl hydrocarbon receptor (AhR; EC50 = 1 μM). |
|---|---|
| Related Catalog |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 499.8±55.0 °C at 760 mmHg |
| Melting Point | 46-49°C |
| Molecular Formula | C15H16Cl3N3O2 |
| Molecular Weight | 376.665 |
| Flash Point | 256.1±31.5 °C |
| Exact Mass | 375.030823 |
| PSA | 47.36000 |
| LogP | 3.98 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.597 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H410 |
| Precautionary Statements | P273-P501 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R50/53 |
| Safety Phrases | S60-S61-S36/37-S26 |
| RIDADR | UN 3077 |
| RTECS | NI4000400 |
| Packaging Group | III |
| HS Code | 2933290012 |
| HS Code | 2933290012 |
|---|---|
| Summary | 2933290012 1-((2-chlorophenyl)diphenylmethyl)-1h-imidazole。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:20.0% |
|
LC-MS analysis of the plasma metabolome--a novel sample preparation strategy.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 978-979 , 83-8, (2015) Blood plasma is a well-known body fluid often analyzed in studies on the effects of toxic compounds as physiological or chemical induced changes in the mammalian body are reflected in the plasma metab... |
|
|
Molecular modelling of the emergence of azole resistance in Mycosphaerella graminicola.
PLoS ONE 6(6) , e20973, (2011) A structural rationale for recent emergence of azole (imidazole and triazole) resistance associated with CYP51 mutations in the wheat pathogen Mycosphaerella graminicola is presented, attained by homo... |
|
|
Human poisoning with prochloraz imidazole fungicide.
Clin. Toxicol. (Phila.) 51(10) , 1237-8, (2013)
|
| Prochloraz |
| Abarit |
| MASTER |
| EINECS 266-994-5 |
| octave |
| N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1-carboxamide |
| FORTAK |
| sprint |
| T5N CNJ AVN3&2OR BG DG FG |
| EYETAK |
| MFCD00078735 |
| P-242 |
| prochloraz [ANSI] |
| KI 835 |
| N-Propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]-1H-imidazole-1-carboxamide |
| MIRAGE |
| ascurit |
| 1H-Imidazole-1-carboxamide, N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]- |
| 1-[N-Propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]carbamoyl]imidazole |
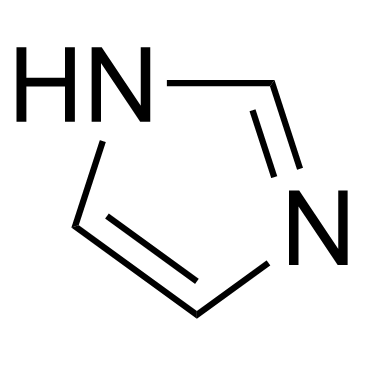 CAS#:288-32-4
CAS#:288-32-4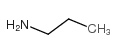 CAS#:107-10-8
CAS#:107-10-8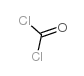 CAS#:75-44-5
CAS#:75-44-5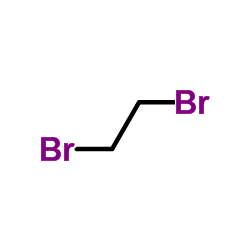 CAS#:106-93-4
CAS#:106-93-4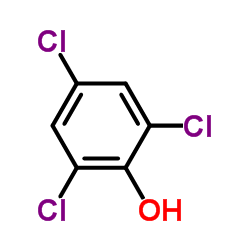 CAS#:88-06-2
CAS#:88-06-2
