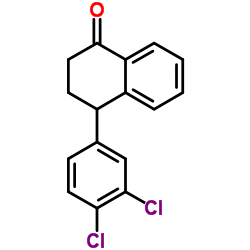
Dasotraline (hydrochloride)

Dasotraline (hydrochloride) structure
|
Common Name | Dasotraline (hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 675126-08-6 | Molecular Weight | 328.664 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H16Cl3N | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Dasotraline (hydrochloride)Dasotraline hydrochloride (SEP-225289 hydrochloride) is a triple reuptake inhibitor that blocks dopamine, norepinephrine, and serotonin transporters with IC50 values of 4, 6, and 11 nM, respectively. |
| Name | (1R,4S)-N-Desmethyl Sertraline Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Dasotraline hydrochloride (SEP-225289 hydrochloride) is a triple reuptake inhibitor that blocks dopamine, norepinephrine, and serotonin transporters with IC50 values of 4, 6, and 11 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 4 nM (dopamine), 6 nM (norepinephrine), 11 nM (serotonin)[1] |
| In Vivo | Acute administration of dasotraline dose-dependently decreases the spontaneous firing rate of LC NE, VTA DA and DR 5-HT neurons through the activation of α2, D2 and 5-HT1A autoreceptors, respectively. Dasotraline predominantly inhibits the firing rate of LC NE neurons while producing only a partial decrease in VTA DA and DR 5-HT neuronal discharge. SEP-225289 is equipotent at inhibiting 5-HT and NE transporters since it prolongs to the same extent the time required for a 50% recovery of the firing activity of dorsal hippocampus CA3 pyramidal neurons from the inhibition induced by microiontophoretic application of 5-HT and NE[1]. Average dopamine and serotonin transporter occupancies increase with increasing doses of SEP-225289. Mean dopamine and serotonin transporter occupancies are 33%±11% and 2%±13%, respectively, for 8 mg; 44%±4% and 9%±10%, respectively, for 12 mg; and 49%±7% and 14%±15%, respectively, for 16 mg[2]. |
| References |
[2]. DeLorenzo C, et al. SEP-225289 serotonin and dopamine transporter occupancy: a PET study. |
| Molecular Formula | C16H16Cl3N |
|---|---|
| Molecular Weight | 328.664 |
| Exact Mass | 327.034821 |
| PSA | 26.02000 |
| LogP | 6.42120 |
|
~94% 
Dasotraline (hy... CAS#:675126-08-6 |
| Literature: Thalen, Lisa K.; Zhao, Dongbo; Sortais, Jean-Baptiste; Paetzold, Jens; Hoben, Christine; Baeckvall, Jan-E. Chemistry - A European Journal, 2009 , vol. 15, # 14 p. 3403 - 3410 |
|
~% 
Dasotraline (hy... CAS#:675126-08-6 |
| Literature: WO2007/6003 A2, ; Page/Page column 26; 29 ; |
|
~% 
Dasotraline (hy... CAS#:675126-08-6 |
| Literature: Organic Process Research and Development, , vol. 11, # 4 p. 726 - 730 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| (1R,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydronaphthalen-1-ylamine hydrochloride |
| UNII:1VIY7J4C0I |
| (1R,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydronaphthalen-1-amine Hydrochloride |
| SEP-225289 HCI |
| (1R,4S)-trans-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenamine HCl |
| trans-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenamine hydrochloride |
| (1R,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenamine hydrochloride (1:1) |
| NorSertraline |
| (1R,4S)-trans 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-napthalenamine hydrochloride |
| (1R,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenamine Hydrochlorid |
| 1-Naphthalenamine, 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-, (1R,4S)-, hydrochloride (1:1) |
| (1R,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenamine Hydrochloride |
| (1R,4S)-N-Desmethyl Sertraline Hydrochloride |
| Dasotraline (hydrochloride) |
![(1R,4S)-N-[4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-naphthalen-1-yl]-formamide structure](https://image.chemsrc.com/caspic/188/675126-11-1.png)