3-nitro-l-tyrosine ethyl ester hydrochloride
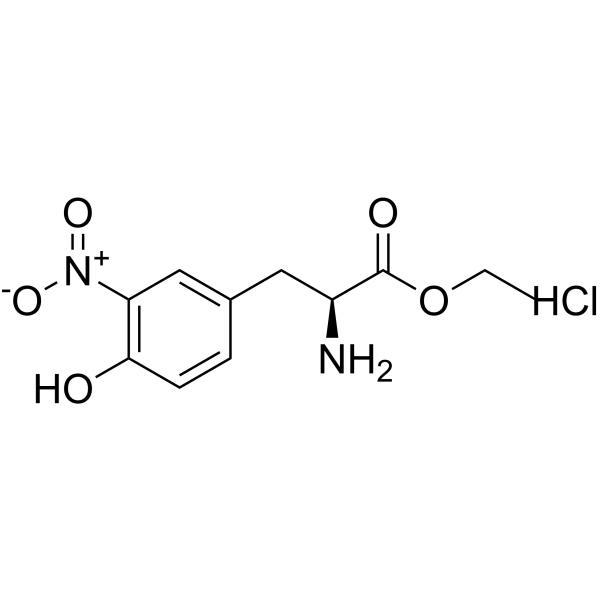
3-nitro-l-tyrosine ethyl ester hydrochloride structure
|
Common Name | 3-nitro-l-tyrosine ethyl ester hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 66737-54-0 | Molecular Weight | 290.70000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H15ClN2O5 | Melting Point | ~185 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of 3-nitro-l-tyrosine ethyl ester hydrochloride3-Nitro-L-tyrosine ethyl ester hydrochloride is a tyrosine derivative[1]. |
| Name | 3-nitro-l-tyrosine ethyl ester hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Nitro-L-tyrosine ethyl ester hydrochloride is a tyrosine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Melting Point | ~185 °C (dec.) |
|---|---|
| Molecular Formula | C11H15ClN2O5 |
| Molecular Weight | 290.70000 |
| Exact Mass | 290.06700 |
| PSA | 118.37000 |
| LogP | 2.75880 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Logic networks based on immunorecognition processes.
J. Phys. Chem. B 113 , 12154-12159, (2009) The biochemical system logically processing biochemical signals using immune-specific and biocatalytic reactions was designed, and the generated output signals were analyzed by AFM and optical means. ... |
|
|
Active site characterization of the single endo-polygalacturonase produced by Fusarium moniliforme NCIM 1276.
Eur. J. Biochem. 268 , 832-840, (2001) Fusarium moniliforme NCIM 1276 isolated from a tropical mangrove ecosystem produces a single extracellular endo-polygalacturonase with an M(r) of 38 kDa and a carbohydrate content of 4%. It has an alk... |
|
|
Structural studies on yeast 3-phosphoglycerate kinase. Identification by immuno-affinity chromatography of one glutamyl residue essential for yeast 3-phosphoglycerate kinase activity. Its location in the primary structure.
Eur. J. Biochem. 105 , 259, (1980) 3-Phosphoglycerate kinase is inactivated by 1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide metho-p-toluenesulfonate and nitrotyrosine ethyl ester. The coupling of 1 mol nitrotyrosine/mol enzyme is suf... |
| 3-Nitro-L-tyrosine ethyl ester hydrochlorideon) |
| ethyl 3-nitro-L-tyrosinate monohydrochloride |
| H-3-NITRO-TYR-OET HCL |