Cefuroxime axetil
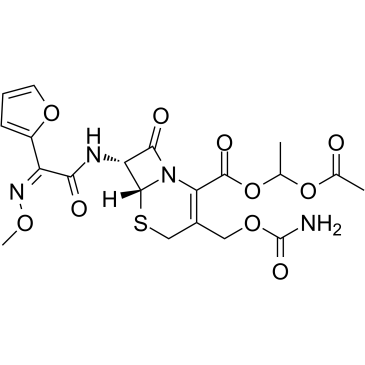
Cefuroxime axetil structure
|
Common Name | Cefuroxime axetil | ||
|---|---|---|---|---|
| CAS Number | 64544-07-6 | Molecular Weight | 510.474 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C20H22N4O10S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Cefuroxime axetilCefuroxime Axetil, a prodrug of the cephalosporin cefuroxime and an oarl broad spectrum antibiotic, inhibits several gram-positive and gram-negative organisms, including those most frequently associated with various common community-acquired infections[1]. |
| Name | Cefuroxime Axetil |
|---|---|
| Synonym | More Synonyms |
| Description | Cefuroxime Axetil, a prodrug of the cephalosporin cefuroxime and an oarl broad spectrum antibiotic, inhibits several gram-positive and gram-negative organisms, including those most frequently associated with various common community-acquired infections[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C20H22N4O10S |
| Molecular Weight | 510.474 |
| Exact Mass | 510.105652 |
| PSA | 214.36000 |
| LogP | 0.85 |
| Index of Refraction | 1.665 |
| InChIKey | KEJCWVGMRLCZQQ-OBEQYXGQSA-N |
| SMILES | CON=C(C(=O)NC1C(=O)N2C(C(=O)OC(C)OC(C)=O)=C(COC(N)=O)CSC12)c1ccco1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2941905990 |
|
~% 
Cefuroxime axetil CAS#:64544-07-6 |
| Literature: US4267320 A1, ; |
|
~% 
Cefuroxime axetil CAS#:64544-07-6 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 83, # 4 p. 553 - 558 |
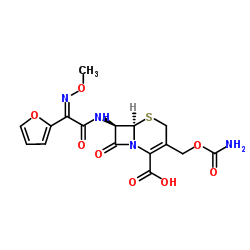
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2941905990 |
|---|
|
Cefuroxime axetil: an updated review of its use in the management of bacterial infections.
Drugs 61(10) , 1455-500, (2001) Cefuroxime axetil, a prodrug of the cephalosporin cefuroxime, has proven in vitro antibacterial activity against several gram-positive and gram-negative organisms, including those most frequently asso... |
|
|
Design and characterization of cefuroxime axetil biphasic floating minitablets.
Drug Deliv. 22(1) , 125-35, (2014) Biphasic floating minitablets of cefuroxime axetil were prepared by melt granulation technique using two different grades of gelucire namely 50/13 and 43/01 to maintain constant plasma drug concentrat... |
|
|
Cefuroxime axetil in the treatment of sinusitis. A review.
Arch. Fam. Med. 3(2) , 165-75, (1994) Cefuroxime axetil is a beta-lactamase-stable, second-generation, oral cephalosporin that penetrates sinus tissue in concentrations exceeding the MIC90 values (the minimum concentration of drug needed ... |
| Ceftin |
| EINECS 259-560-1 |
| Cefuroxime axetil |
| cefuroxime 1-acetoxyethyl ester |
| Zinat |
| UNII:Z49QDT0J8Z |
| 1-Acetoxyethyl (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-(2-furyl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| 1-(acetyloxy)ethyl (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Zinnat |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-[[(2Z)-2-(2-furanyl)-2-(methoxyimino)-1-oxoethyl]amino]-8-oxo-, 1-(acetyloxy)ethyl ester, (6R,7R)- |
| Cetoxil |
| MFCD00864889 |
| Cefuroximeaxetil |
![5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-[[(2E)-2-(2-furanyl)-2-(methoxyimino)acetyl]amino]-8-oxo-, 1-(acetyloxy)ethyl ester, (6R,7R)- structure](https://image.chemsrc.com/caspic/177/97232-96-7.png)

