Isovanillic acid
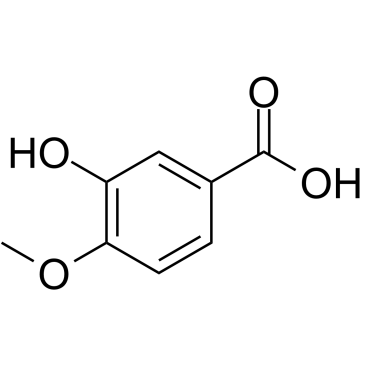
Isovanillic acid structure
|
Common Name | Isovanillic acid | ||
|---|---|---|---|---|
| CAS Number | 645-08-9 | Molecular Weight | 168.147 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 344.3±27.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O4 | Melting Point | 250-253 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.2±17.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Isovanillic acidIsovanillic acid (3-Hydroxy-4-methoxybenzoic acid) is a phenolic acid isolated from isolated from S. frutescens, with Anti-inflammatory activity[1]. |
| Name | 3-hydroxy-4-methoxybenzoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Isovanillic acid (3-Hydroxy-4-methoxybenzoic acid) is a phenolic acid isolated from isolated from S. frutescens, with Anti-inflammatory activity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 344.3±27.0 °C at 760 mmHg |
| Melting Point | 250-253 °C(lit.) |
| Molecular Formula | C8H8O4 |
| Molecular Weight | 168.147 |
| Flash Point | 145.2±17.2 °C |
| Exact Mass | 168.042252 |
| PSA | 66.76000 |
| LogP | 1.35 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.586 |
| InChIKey | LBKFGYZQBSGRHY-UHFFFAOYSA-N |
| SMILES | COc1ccc(C(=O)O)cc1O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | BZ4850000 |
| HS Code | 2918990090 |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Structure-Activity Relationships of Antimicrobial Gallic Acid Derivatives from Pomegranate and Acacia Fruit Extracts against Potato Bacterial Wilt Pathogen.
Chem. Biodivers. 12 , 955-62, (2015) Bacterial wilts of potato, tomato, pepper, and or eggplant caused by Ralstonia solanacearum are among the most serious plant diseases worldwide. In this study, the issue of developing bactericidal age... |
|
|
Mass spectrometric behavior of phenolic acids standards and their analysis in the plant samples with LC/ESI/MS system.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 967 , 21-7, (2014) Liquid chromatography coupled to mass spectrometry (MS) with electrospray ionization (ESI) is one of analytical techniques to obtain accurate results of low molecular weight aromatic compounds in biol... |
|
|
Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes.
Mol. Nutr. Food. Res. 59 , 1143-54, (2015) Flavonoids are generally studied in vitro, in isolation, and as unmetabolized precursor structures. However, in the habitual diet, multiple flavonoids are consumed together and found present in the ci... |
| Isovanillic acid |
| Benzoic acid, 3-hydroxy-4-methoxy- |
| MFCD00002507 |
| p-Anisic acid,3-hydroxy |
| 3-hydroxy-4-methoxy-benzoic acid |
| isovanilinic acid |
| 3-Hydroxyanisic acid |
| EINECS 211-430-5 |
| 4-methoxy-protocatechuic acid |
| 3-Hydroxy-4-methoxybenzoic acid |
| Acide isovanillique |
| 3-hydroxy-4-methoxybenzoate |
| 3-Hydroxy-p-anisic acid |

