Boc-6-aminohexanoic acid

Boc-6-aminohexanoic acid structure
|
Common Name | Boc-6-aminohexanoic acid | ||
|---|---|---|---|---|
| CAS Number | 6404-29-1 | Molecular Weight | 231.289 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 380.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C11H21NO4 | Melting Point | 35-40 °C | |
| MSDS | USA | Flash Point | 183.8±23.2 °C | |
Use of Boc-6-aminohexanoic acidBoc-6-aminohexanoic acid is an alkyl/ether-based PROTAC linker that can be used in the synthesis of PROTACs[1]. |
| Name | Boc-6-aminohexanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-6-aminohexanoic acid is an alkyl/ether-based PROTAC linker that can be used in the synthesis of PROTACs[1]. |
|---|---|
| Related Catalog | |
| Target |
Alkyl/ether |
| In Vitro | PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 380.3±25.0 °C at 760 mmHg |
| Melting Point | 35-40 °C |
| Molecular Formula | C11H21NO4 |
| Molecular Weight | 231.289 |
| Flash Point | 183.8±23.2 °C |
| Exact Mass | 231.147064 |
| PSA | 75.63000 |
| LogP | 1.67 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.464 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S22-S24/25-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924199090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
The literature on affinity chromatography.
Meth. Enzymol. 34 , 3, (1974)
|
|
|
Affinity chromatography of carboxypeptidase B.
Meth. Enzymol. 34 , 411, (1974)
|
|
|
Synthesis and properties of radioiodinated phospholipid analogues that spontaneously undergo vesicle-vesicle and vesicle-cell transfer.
Biochemistry 22 , 3617, (1983) An efficient method for the synthesis and purification of a variety of iodinated phospholipid analogues is described. 1-Acyl-2-[[[3-(3-[125I]iodo-4-hydroxyphenyl)- propionyl]amino]caproyl]phosphatidyl... |
| 6-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)hexanoic acid |
| 6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid |
| 6-[(tert-butoxycarbonyl)amino]hexanoic acid |
| Hexanoic acid, 6-[[(1,1-dimethylethoxy)carbonyl]amino]- |
| MFCD00037798 |
| Boc-ε-Acp-OH |
| Boc-ε-Ahx-OH |
 CAS#:24424-99-5
CAS#:24424-99-5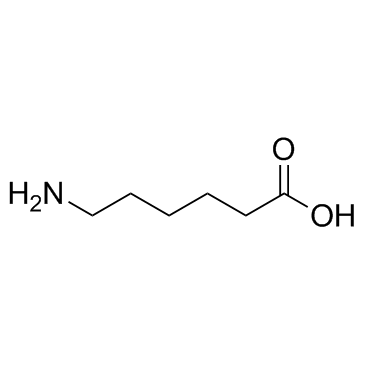 CAS#:60-32-2
CAS#:60-32-2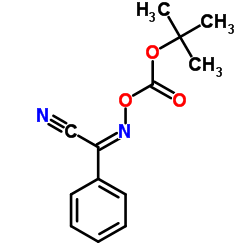 CAS#:58632-95-4
CAS#:58632-95-4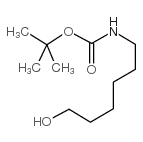 CAS#:75937-12-1
CAS#:75937-12-1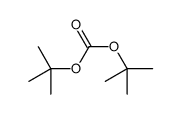 CAS#:34619-03-9
CAS#:34619-03-9 CAS#:74651-77-7
CAS#:74651-77-7 CAS#:1070-19-5
CAS#:1070-19-5 CAS#:80860-42-0
CAS#:80860-42-0 CAS#:7646-93-7
CAS#:7646-93-7 CAS#:38822-56-9
CAS#:38822-56-9![6-[[(1,1-Dimethylethoxy)carbonyl]amino]hexanoic acid 2,5-dioxo-1-pyrrolidinyl ester structure](https://image.chemsrc.com/caspic/026/51513-80-5.png) CAS#:51513-80-5
CAS#:51513-80-5 CAS#:105-60-2
CAS#:105-60-2 CAS#:51857-17-1
CAS#:51857-17-1 CAS#:142356-33-0
CAS#:142356-33-0 CAS#:35408-82-3
CAS#:35408-82-3 CAS#:1926-80-3
CAS#:1926-80-3 CAS#:202925-92-6
CAS#:202925-92-6
