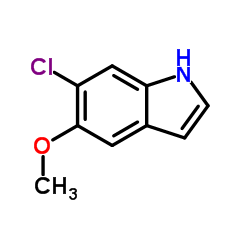
6-Chloromelatonin
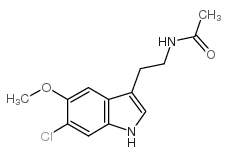
6-Chloromelatonin structure
|
Common Name | 6-Chloromelatonin | ||
|---|---|---|---|---|
| CAS Number | 63762-74-3 | Molecular Weight | 266.72300 | |
| Density | 1.272g/cm3 | Boiling Point | 536.7ºC at 760 mmHg | |
| Molecular Formula | C13H15ClN2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 278.4ºC | |
Use of 6-Chloromelatonin6-Chloromelatonin is a potent melatonin receptor agonist with greater metabolic stability than melatonin. 6-Chloromelatonin compete for [3H]-melatonin and 2-[125I]-iodomelatonin binding to MT1 receptors (pKi=8.9 and 9.1, respectively). 6-Chloromelatonin compete for [3H]-melatonin binding to MT2 receptors (pKi=9.77)[1][2]. |
| Name | N-[2-(6-chloro-5-methoxy-1H-indol-3-yl)ethyl]acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Chloromelatonin is a potent melatonin receptor agonist with greater metabolic stability than melatonin. 6-Chloromelatonin compete for [3H]-melatonin and 2-[125I]-iodomelatonin binding to MT1 receptors (pKi=8.9 and 9.1, respectively). 6-Chloromelatonin compete for [3H]-melatonin binding to MT2 receptors (pKi=9.77)[1][2]. |
|---|---|
| Related Catalog | |
| Target |
MT1:8.9 (pKi) MT2:9.1 (pKi) |
| In Vitro | 6-Chloromelatonin competes for [3H]-melatonin binding sites in human platelet (Ki=11.4 nM)[3]. 6-chloromelatonin (10 pM, 1 nM, 100 nM, 10 μM; 72 hours) inhibits, in a dose-dependent manner, forskolin-stimulated hCG-beta secretion in JEG-3 and BeWo cells but had no effect on basal human chorionic gonadotrophin (hCG-beta) levels[4]. |
| In Vivo | Rats injected with the melatonin agonist, 6-chloromelatonin (0.5 mg/kg) on the day after the phase shift has markedly higher excretion rates of 6-sulphatoxymelatonin compared to those of the controls[5]. |
| References |
| Density | 1.272g/cm3 |
|---|---|
| Boiling Point | 536.7ºC at 760 mmHg |
| Molecular Formula | C13H15ClN2O2 |
| Molecular Weight | 266.72300 |
| Flash Point | 278.4ºC |
| Exact Mass | 266.08200 |
| PSA | 54.12000 |
| LogP | 2.89940 |
| Index of Refraction | 1.608 |
| InChIKey | LUINDDOUWHRIPW-UHFFFAOYSA-N |
| SMILES | COc1cc2c(CCNC(C)=O)c[nH]c2cc1Cl |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T |
|---|---|
| Risk Phrases | 60-23/24/25-36/37/38 |
| Safety Phrases | 53-26-36/37/39-45 |
| HS Code | 2933990090 |
|
~% 
6-Chloromelatonin CAS#:63762-74-3 |
| Literature: Huegel, Helmut M. Synthesis, 1983 , # 11 p. 935 - 936 |
|
~% 
6-Chloromelatonin CAS#:63762-74-3 |
| Literature: Huegel, Helmut M. Synthesis, 1983 , # 11 p. 935 - 936 |
|
~% 
6-Chloromelatonin CAS#:63762-74-3 |
| Literature: Huegel, Helmut M. Synthesis, 1983 , # 11 p. 935 - 936 |
|
~% 
6-Chloromelatonin CAS#:63762-74-3 |
| Literature: Huegel, Helmut M. Synthesis, 1983 , # 11 p. 935 - 936 |
|
~% 
6-Chloromelatonin CAS#:63762-74-3 |
| Literature: Huegel, Helmut M. Synthesis, 1983 , # 11 p. 935 - 936 |
|
~% 
6-Chloromelatonin CAS#:63762-74-3 |
| Literature: Huegel, Helmut M. Synthesis, 1983 , # 11 p. 935 - 936 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Tocris-0443 |
| 6-CHLOROMELATONIN |
| 6-Cl-melatonin |
| Lopac-C-0331 |

![2-chloro-5-[(β-dimethylamino)-vinyl]-4-nitroanisole structure](https://image.chemsrc.com/caspic/388/63762-71-0.png)