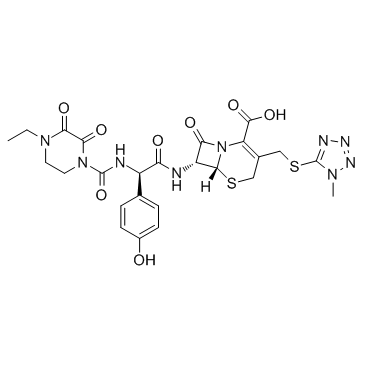
Cefoperazone sodium
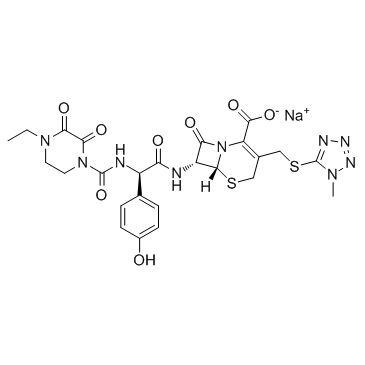
Cefoperazone sodium structure
|
Common Name | Cefoperazone sodium | ||
|---|---|---|---|---|
| CAS Number | 62893-20-3 | Molecular Weight | 667.649 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H26N9NaO8S2 | Melting Point | 200-202°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of Cefoperazone sodiumCefoperazone sodium salt is a cephalosporin antibiotic for inhibition of rMrp2-mediated [3H]E217βG uptake with IC50 of 199 μM.Target: AntibacterialCefoperazone is a sterile, semisynthetic, broad-spectrum, parenteral cephalosporin antibiotic for intravenous or intramuscular administration. After intravenous administration of 2 g of Cefoperazone, levels in serum rang from 202μg/mL to 375 μg/mL depending on the period of drug administration. After intramuscular injection of 2 g of Cefoperazone, the mean peak serum level is 111 μg/mL at 1.5 hours. At 12 hours after dosing, mean serum levels are still 2 to 4 μg/mL. Cefoperazone is 90% bound to serum proteins. |
| Name | sodium,(6R,7R)-7-[[(2R)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-(4-hydroxyphenyl)acetyl]amino]-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Cefoperazone sodium salt is a cephalosporin antibiotic for inhibition of rMrp2-mediated [3H]E217βG uptake with IC50 of 199 μM.Target: AntibacterialCefoperazone is a sterile, semisynthetic, broad-spectrum, parenteral cephalosporin antibiotic for intravenous or intramuscular administration. After intravenous administration of 2 g of Cefoperazone, levels in serum rang from 202μg/mL to 375 μg/mL depending on the period of drug administration. After intramuscular injection of 2 g of Cefoperazone, the mean peak serum level is 111 μg/mL at 1.5 hours. At 12 hours after dosing, mean serum levels are still 2 to 4 μg/mL. Cefoperazone is 90% bound to serum proteins. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 200-202°C |
|---|---|
| Molecular Formula | C25H26N9NaO8S2 |
| Molecular Weight | 667.649 |
| Exact Mass | 667.124329 |
| PSA | 273.69000 |
| Water Solubility | H2O: 50 mg/mL, clear, faintly yellow |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P342 + P311 |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R42/43 |
| Safety Phrases | S22-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | XI0374000 |
| HS Code | 2941905600 |
|
~97% 
Cefoperazone sodium CAS#:62893-20-3 |
| Literature: DSM SINOCHEM PHARMACEUTICALS NETHERLANDS B.V.; MAO, Chang-Long; XU, Jian-Jun; SONG, Bo Patent: WO2014/12849 A1, 2014 ; Location in patent: Page/Page column 6; 7 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2941905600 |
|---|
|
Housing conditions modulate the severity of Mycoplasma pulmonis infection in mice deficient in class A scavenger receptor.
Comp. Med. 64(6) , 424-39, (2014) Mycoplasmosis is a frequent causative microbial agent of community-acquired pneumonia and has been linked to exacerbation of chronic obstructive pulmonary disease. The macrophage class A scavenger rec... |
|
|
Randomized comparison of piperacillin-tazobactam plus amikacin versus cefoperazone-sulbactam plus amikacin for management of febrile neutropenia in children with lymphoma and solid tumors.
Pediatr. Hematol. Oncol. 30(2) , 141-8, (2013) The objective of this study was to compare the effectiveness of piperacillin-tazobactam (PIP/TAZO) plus amikacin (AMK) (PIP/TAZO+AMK) versus cefoperazone-sulbactam (CS) plus AMK (CS+AMK) for the treat... |
|
|
Interaction between caspofungin or voriconazole and cefoperazone-sulbactam or piperacillin-tazobactam by in vitro and in vivo methods.
APMIS 122(5) , 412-7, (2014) Immunosuppressive patients are at risk of fungal and bacterial infections. Therefore, these patients receive prophylactic, preemptive, empirical or target antifungal and concomitant antibiotic therapy... |
| Cefoperazone sodium |
| MFCD07793331 |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2R)-2-[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, sodium salt, (6R,7R)- (1:1) |
| Sodium (6R,7R)-7-{[(2R)-2-{[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino}-2-(4-hydroxyphenyl)acetyl]amino}-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Sodium (6R,7R)-7-{[(2R)-2-{[(4-ethyl-2,3-dioxopiperazin-1-yl)carbonyl]amino}-2-(4-hydroxyphenyl)acetyl]amino}-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| cefoperazone-Na |
| Natrium-(6R,7R)-7-{[(2R)-2-{[(4-ethyl-2,3-dioxopiperazin-1-yl)carbonyl]amino}-2-(4-hydroxyphenyl)acetyl]amino}-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylat |
| Sodium cefoperazone |
| cefoperazone monosodium |
| EINECS 263-751-5 |
| Tomabef |
| cephoperazone sodium |
| Cefoperazone sodium [USAN:JAN] |
| Cefoperazone sodium salt |
| CEFOBID IN PLASTIC CONTAINER |
| T 1551 |
| Cefoperazone sodium (JP15/USP) |
| Cefoperazonesodium |
| Cefoperazone (sodium salt) |