Proadifen hydrochloride
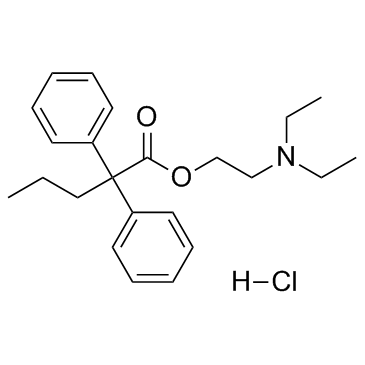
Proadifen hydrochloride structure
|
Common Name | Proadifen hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 62-68-0 | Molecular Weight | 389.959 | |
| Density | N/A | Boiling Point | 460.8ºC at 760 mmHg | |
| Molecular Formula | C23H32ClNO2 | Melting Point | 122-123ºC | |
| MSDS | Chinese USA | Flash Point | 132.3ºC | |
Use of Proadifen hydrochlorideProadifen hydrochloride is a Cytochrome P450 inhibitor (IC50 = 19μM). IC50 value: 19μMTarget: P450Proadifen HCl has many biochemical functions, some of which include: inhibitory effects on NOS1 (neuronal nitric oxide synthase; IC50 = 90 mM), adult mouse skeletal muscle AChR (acetyl choline receptor), hepatic drug metabolism via the CYP (cytochrome P450) system, CYP-dependent (cytochrome P450-dependent) arachidonate metabolism (90% at 50 μM), transmembrane calcium influx, and platelet thromboxane synthesis. This compound has also been shown to block KIR6.1 (ATP-sensitive inward rectifier potassium channel 8; IC50 = 4.4 mM) and stimulate endothelial cell prostacyclin production. |
| Name | 2-(diethylamino)ethyl 2,2-diphenylpentanoate,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Proadifen hydrochloride is a Cytochrome P450 inhibitor (IC50 = 19μM). IC50 value: 19μMTarget: P450Proadifen HCl has many biochemical functions, some of which include: inhibitory effects on NOS1 (neuronal nitric oxide synthase; IC50 = 90 mM), adult mouse skeletal muscle AChR (acetyl choline receptor), hepatic drug metabolism via the CYP (cytochrome P450) system, CYP-dependent (cytochrome P450-dependent) arachidonate metabolism (90% at 50 μM), transmembrane calcium influx, and platelet thromboxane synthesis. This compound has also been shown to block KIR6.1 (ATP-sensitive inward rectifier potassium channel 8; IC50 = 4.4 mM) and stimulate endothelial cell prostacyclin production. |
|---|---|
| Related Catalog |
| Boiling Point | 460.8ºC at 760 mmHg |
|---|---|
| Melting Point | 122-123ºC |
| Molecular Formula | C23H32ClNO2 |
| Molecular Weight | 389.959 |
| Flash Point | 132.3ºC |
| Exact Mass | 389.212158 |
| PSA | 29.54000 |
| LogP | 5.45980 |
| InChIKey | FHIKZROVIDCMJA-UHFFFAOYSA-N |
| SMILES | CCCC(C(=O)OCCN(CC)CC)(c1ccccc1)c1ccccc1.Cl |
| Storage condition | −20°C |
| Stability | Store in Freezer |
| Water Solubility | Soluble in methanol or water |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
Effects of cytochrome P450 inhibitors on peroxidase activity.
Neuro Endocrinol. Lett. 33 Suppl 3 , 33-40, (2012) Of several enzymes metabolizing xenobiotics, cytochrome P450 (CYP) and peroxidase enzymes seem to be most important. One of the major challenges in studies investigating metabolism of xenobiotics is t... |
|
|
Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis.
PLoS ONE 10(2) , e0116961, (2015) Methamphetamine is a highly addictive psychostimulant that causes profound damage to the brain and other body organs. Post mortem studies of human tissues have linked the use of this drug to diseases ... |
|
|
Subepithelial trypsin induces enteric nerve-mediated anion secretion by activating proteinase-activated receptor 1 in the mouse cecum.
J. Physiol. Sci. 62(3) , 211-9, (2012) Serine proteases are versatile signaling molecules and often exert this function by activating the proteinase-activated receptors (PAR(1)-PAR(4)). Our previous study on the mouse cecum has shown that ... |
| Benzeneacetic acid, α-phenyl-α-propyl-, 2-(diethylamino)ethyl ester, hydrochloride (1:1) |
| 2-Diethylaminoethyl Propyldiphenylacetate Hydrochloride |
| β-Diethylaminoethyl diphenylpropylacetate hydrochloride |
| F 525-A |
| SKF-525A,Hydrochloride |
| RP 5171 |
| 2-Diethylaminoethyl α,α-diphenyl valerate hydrochloride |
| 2,2-Diphenylvaleric Acid 2-(Diethylamino)ethyl Ester Hydrochloride |
| 2-[(2,2-diphenylpentanoyl)oxy]-N,N-diethylethanaminium chloride |
| Benzeneacetic acid, α-phenyl-α-propyl-, 2- (diethylamino)ethyl ester, hydrochloride |
| Proadifen HCl |
| Proadifen hydrochloride |
| proadifen |
| 2-Diethylaminoethyl-2,2-diphenylvalerate Hydrochloride |
| N,N-diethylaminoethyl-2,2-diphenylvalerate hydrochloride |
| Valeric acid, 2,2-diphenyl-, 2-diethylaminoethyl ester, hydrochloride |
| SKF 525A |
| Valeric acid, 2,2-diphenyl-, 2- (diethylamino)ethyl ester hydrochloride |
| Benzeneacetic acid, α-phenyl-α-propyl-, 2-(diethylamino)ethyl ester hydrochloride |
| 2-diethylaminoethyl 2,2-diphenylvalerate hydrochloride |
| MFCD00055151 |
| Propyladiphenin |
| 2-(Diethylamino)ethyl 2,2-diphenylpentanoate hydrochloride (1:1) |
| a-Phenyl-a-propylbenzeneacetic Acid 2-(Diethylamino)ethyl Ester Hydrochloride |
| SKF-525A |

