HZ-166
Modify Date: 2025-08-25 20:39:29
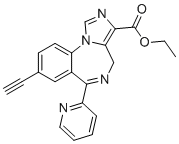
HZ-166 structure
|
Common Name | HZ-166 | ||
|---|---|---|---|---|
| CAS Number | 612527-56-7 | Molecular Weight | 356.385 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H16N4O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of HZ-166HZ-166 (HZ166) is a GABAA receptor subtype-selective benzodiazepine site ligand with preferential activity at α2- and α3-GABA(A) receptors; displays a statistically significant higher affinity for receptors not containing the α1 subunit with a rank order of α5 (Ki=140nM) > α2 (Ki=269 nM) > α1 (Ki=382 nM); The Ki value of HZ166 for the α3β3γ2 combination (185± 47 nM) was statistically significantly lower than the Ki value observed for α1β3γ2 but not different from those of α2β3γ2 and α5β3γ2; HZ-166 is antihyperalgesic in mouse models of inflammatory and neuropathic pain. |
| Name | HZ-166 |
|---|
| Description | HZ-166 (HZ166) is a GABAA receptor subtype-selective benzodiazepine site ligand with preferential activity at α2- and α3-GABA(A) receptors; displays a statistically significant higher affinity for receptors not containing the α1 subunit with a rank order of α5 (Ki=140nM) > α2 (Ki=269 nM) > α1 (Ki=382 nM); The Ki value of HZ166 for the α3β3γ2 combination (185± 47 nM) was statistically significantly lower than the Ki value observed for α1β3γ2 but not different from those of α2β3γ2 and α5β3γ2; HZ-166 is antihyperalgesic in mouse models of inflammatory and neuropathic pain. |
|---|---|
| References | References 1. Fischer BD, et al. Neuropharmacology. 2010 Dec;59(7-8):612-8. 2. Di Lio A, et al. Neuropharmacology. 2011 Mar;60(4):626-32. 3. Witkin JM, et al. Neuropharmacology. 2018 Jul 15;137:332-343. 4. Rivas FM, et al. J Med Chem. 2009 Apr 9;52(7):1795-8. View Related Products by Target GABA Receptor |
| Molecular Formula | C21H16N4O2 |
|---|---|
| Molecular Weight | 356.385 |