7-Methylquinoline
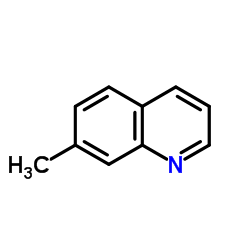
7-Methylquinoline structure
|
Common Name | 7-Methylquinoline | ||
|---|---|---|---|---|
| CAS Number | 612-60-2 | Molecular Weight | 143.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 254.5±9.0 °C at 760 mmHg | |
| Molecular Formula | C10H9N | Melting Point | 35-37 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 104.4±11.3 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
| Name | 7-Methylquinoline |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 254.5±9.0 °C at 760 mmHg |
| Melting Point | 35-37 °C(lit.) |
| Molecular Formula | C10H9N |
| Molecular Weight | 143.185 |
| Flash Point | 104.4±11.3 °C |
| Exact Mass | 143.073502 |
| PSA | 12.89000 |
| LogP | 2.54 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.625 |
| InChIKey | KDYVCOSVYOSHOL-UHFFFAOYSA-N |
| SMILES | Cc1ccc2cccnc2c1 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. May be light sensitive. |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R37/38;R41 |
| Safety Phrases | S26-S39 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | VC0560000 |
| Packaging Group | I; II; III |
| Hazard Class | 6.1 |
| HS Code | 2933499090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
On the metabolism of quinoline and isoquinoline: possible molecular basis for differences in biological activities.
Carcinogenesis 4 , 1169-1173, (1983) Quinoline is a hepatocarcinogen in mice and rats, a mutagen in Salmonella typhimurium, and induces unscheduled DNA synthesis in primary cultures of rat hepatocytes. In contrast, isoquinoline has not b... |
|
|
88. The Skraup reaction with m-substituted anilines. Bradford L, et al.
J. Chem. Soc. , 437-45, (1947)
|
| Quinoline, 7-methyl- |
| Quinoline,7-methyl |
| 7-Methylquinoline |
| EINECS 210-316-2 |
| MFCD00006805 |
| 7-aminoquinoline |
| 7-Methyl-chinolin |
| 7-methyl-Quinoline |
| m-Toluquinoline |
 CAS#:58960-03-5
CAS#:58960-03-5 CAS#:99-08-1
CAS#:99-08-1 CAS#:156-87-6
CAS#:156-87-6 CAS#:56-81-5
CAS#:56-81-5 CAS#:108-44-1
CAS#:108-44-1![3-[bis(3-hydroxypropyl)amino]propan-1-ol Structure](https://image.chemsrc.com/caspic/429/14002-34-7.png) CAS#:14002-34-7
CAS#:14002-34-7 CAS#:1504-75-2
CAS#:1504-75-2![N-[3-m-tolylamino]-2-propenylidene]-m-toluidine Structure](https://image.chemsrc.com/caspic/400/23545-77-9.png) CAS#:23545-77-9
CAS#:23545-77-9 CAS#:107-18-6
CAS#:107-18-6 CAS#:49573-30-0
CAS#:49573-30-0 CAS#:5470-82-6
CAS#:5470-82-6 CAS#:14061-25-7
CAS#:14061-25-7 CAS#:27356-39-4
CAS#:27356-39-4 CAS#:7471-63-8
CAS#:7471-63-8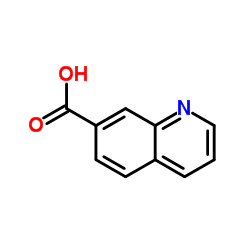 CAS#:1078-30-4
CAS#:1078-30-4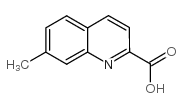 CAS#:75434-10-5
CAS#:75434-10-5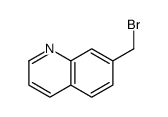 CAS#:769100-08-5
CAS#:769100-08-5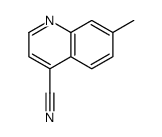 CAS#:854864-06-5
CAS#:854864-06-5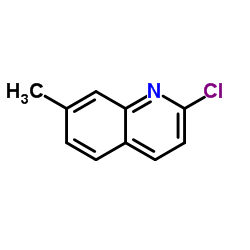 CAS#:4295-12-9
CAS#:4295-12-9
