2-Nitrophenylacetonitrile
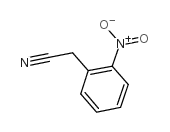
2-Nitrophenylacetonitrile structure
|
Common Name | 2-Nitrophenylacetonitrile | ||
|---|---|---|---|---|
| CAS Number | 610-66-2 | Molecular Weight | 162.14500 | |
| Density | 1.272 g/cm3 | Boiling Point | 178°C | |
| Molecular Formula | C8H6N2O2 | Melting Point | 82-85 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 138ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Nitrophenylacetonitrile |
|---|---|
| Synonym | More Synonyms |
| Density | 1.272 g/cm3 |
|---|---|
| Boiling Point | 178°C |
| Melting Point | 82-85 °C(lit.) |
| Molecular Formula | C8H6N2O2 |
| Molecular Weight | 162.14500 |
| Flash Point | 138ºC |
| Exact Mass | 162.04300 |
| PSA | 69.61000 |
| LogP | 2.18408 |
| Index of Refraction | 1.577 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | <0.01 g/100 mL at 20 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H315-H319-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | 3439 |
| WGK Germany | 3 |
| RTECS | AM1248000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2926909090 |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Enantioselective total synthesis of (-)-strychnine using the catalytic asymmetric Michael reaction and tandem cyclization.
J. Am. Chem. Soc. 124(49) , 14546-7, (2002) The enantioselective total synthesis of (-)-strychnine was accomplished through the use of the highly practical catalytic asymmetric Michael reaction (0.1 mol % of (R)-ALB, more than kilogram scale, w... |
|
|
Rapid access to α-carbolines via a one-pot tandem reaction of α,β-unsaturated ketones with 2-nitrophenylacetonitrile and the anti-proliferative activities of the products.
Org. Biomol. Chem. 12(2) , 355-61, (2014) An effective and convenient method has been developed for the preparation of 2 or 2,4-substituted α-carbolines via a one-pot tandem reaction of α,β-unsaturated ketones with 2-nitrophenylacetonitrile i... |
| MFCD00007183 |
| 2-(2-nitrophenyl)acetonitrile |
| EINECS 210-231-0 |

