Amrinone

Amrinone structure
|
Common Name | Amrinone | ||
|---|---|---|---|---|
| CAS Number | 60719-84-8 | Molecular Weight | 187.198 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 451.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C10H9N3O | Melting Point | 294-297ºC | |
| MSDS | Chinese USA | Flash Point | 226.9±28.7 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of AmrinoneAmrinone (Inamrinone) is a positive inotropic-vasodilator agent. Amrinone is a selective phosphodiesterase III inhibitor that increases cyclic adenosine monophosphate by preventing its breakdown. Amrinone is also an orally active, non-glycosidic and non-catecholamine cardiotonic agent[1][2][3]. |
| Name | 3-amino-5-pyridin-4-yl-1H-pyridin-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | Amrinone (Inamrinone) is a positive inotropic-vasodilator agent. Amrinone is a selective phosphodiesterase III inhibitor that increases cyclic adenosine monophosphate by preventing its breakdown. Amrinone is also an orally active, non-glycosidic and non-catecholamine cardiotonic agent[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Amrinone (Inamrinone) produced a dose-dependent inhibition of ADP-induced rat platelet aggregation in vitro as well as ex vivo in rats. The proliferation of human aortic smooth muscle cells in culture stimulated with FBS or PDGF was also inhibited by amrinone[4]. |
| In Vivo | Amrinone (Inamrinone) is administered subcutaneously to rats at a dose of 10 mg/kg/day for 14 days, significant reduction of neointimal thickness was noted[4]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 451.5±45.0 °C at 760 mmHg |
| Melting Point | 294-297ºC |
| Molecular Formula | C10H9N3O |
| Molecular Weight | 187.198 |
| Flash Point | 226.9±28.7 °C |
| Exact Mass | 187.074554 |
| PSA | 71.77000 |
| LogP | -0.54 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.625 |
| InChIKey | RNLQIBCLLYYYFJ-UHFFFAOYSA-N |
| SMILES | Nc1cc(-c2ccncc2)c[nH]c1=O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | S28-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | DW2500000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29333999 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
A phosphodiesterase III inhibitor protects rat liver from sinusoidal obstruction syndrome through heme oxygenase-1 induction.
Ann. Surg. 251(4) , 780; author reply 780, (2010)
|
|
|
Chronotropic and inotropic actions of amrinone, carbazeran and isobutylmethyl xanthine: role of phosphodiesterase inhibition.
Br. J. Pharmacol. 98 , 291-301, (1999) 1. The chronotropic and inotropic effects of amrinone, carbazeran and 3-isobutyl-1-methyl xanthine (IBMX) were examined in isolated preparations of papillary muscle and right atria from rabbit heart. ... |
|
|
Design, synthesis and pharmacological evaluation of 6-hydroxy-4-methylquinolin-2(1H)-one derivatives as inotropic agents.
J. Enzyme Inhib. Med. Chem. 24(4) , 918-29, (2009) Selective PDE3 inhibitors improve cardiac contractility and may be used in congestive heart failure. However, their proarrhythmic potential is the most important side effect. In this research we desig... |
| Amrinona |
| Cordemcura |
| Wincoram |
| Amrinon |
| Amrinone |
| 5-Amino-3,4'-bipyridin-6-ol |
| [3,4'-Bipyridin]-6(1H)-one, 5-amino- |
| Inocor |
| MFCD00083228 |
| [3,4'-bipyridin]-6-ol, 5-amino- |
| Inamrinone |
| Amrinonum |
| Amcoral |
| EINECS 262-390-0 |
| (3,4'-Bipyridin)-6(1H)-one, 5-amino- |
| 5-Amino-3,4'-bipyridin-6(1H)-one |
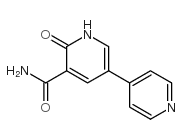 CAS#:62749-46-6
CAS#:62749-46-6![5-nitro-1H-[3,4']bipyridinyl-6-one Structure](https://image.chemsrc.com/caspic/305/62749-33-1.png) CAS#:62749-33-1
CAS#:62749-33-1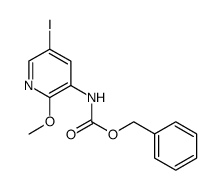 CAS#:893440-43-2
CAS#:893440-43-2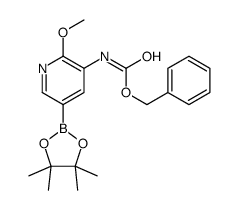 CAS#:893440-45-4
CAS#:893440-45-4
