sulfasalazine
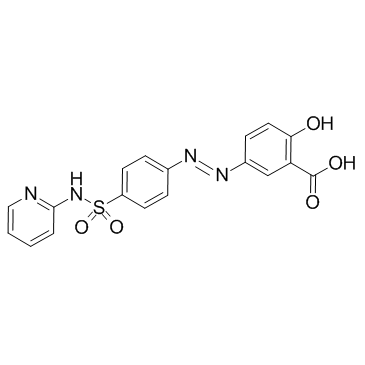
sulfasalazine structure
|
Common Name | sulfasalazine | ||
|---|---|---|---|---|
| CAS Number | 599-79-1 | Molecular Weight | 398.393 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 689.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C18H14N4O5S | Melting Point | 260-265 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 370.7±34.3 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of sulfasalazineSulfasalazine is a drug for the treatment of rheumatoid arthritis and ulcerative colitis. Sulfasalazine is reported to suppress NF-κB activity. |
| Name | sulfasalazine |
|---|---|
| Synonym | More Synonyms |
| Description | Sulfasalazine is a drug for the treatment of rheumatoid arthritis and ulcerative colitis. Sulfasalazine is reported to suppress NF-κB activity. |
|---|---|
| Related Catalog | |
| Target |
RelA Autophagy |
| In Vitro | Treatment of SW620 colon cells with sulfasalazine inhibits TNFα-, LPS-, or phorbol ester-induced NFκB activation. NFκB–dependent transcription is inhibited by sulfasalazine at micro- to millimolar concentrations. TNFα-induced nuclear translocation of NFκB is prevented by sulfasalazine through inhibition of IκBα degradation[1]. Pre‐incubation with 5 mM of sulfasalazine alone significantly increases basal mRNA expression of all pro‐inflammatory cytokines with levels of IL‐6 mRNA increased by 80‐fold compared with vehicle control[2]. Once digested, sulfasalazine is cleaved into sulfapyridine and 5-aminosalicylic acid by colonic bacteria, and the latter, too, is reported to suppress NF-kappaB activity[3]. |
| In Vivo | At low doses (0.25 mM), SAS is able to suppress glioma growth by over 60% compared to untreated controls[3]. |
| Cell Assay | Sulfasalazine is dissolved in culture medium. SW620 cells are grown in Dulbecco’s modified Eagle medium, supplemented with 10% heat-inactivated FCS, 2 mmol/liter glutamine, and 1% (wt/vol) penicillin/streptomycin. SW620 cells are transfected with the 3xIgkBLuc reporter construct. After 18 h, cells are incubated with either medium alone or with sulfasalazine (0.1, 0.2, 0.5, 1, 2, 5 mM) before stimulation with TNFα, LPS, or PMA. Luciferase assay is performed[1]. |
| Animal Admin | Mice: Sulfasalazine is dissolved in 0.1 M NaOH, and then neutralized by titrating with 0.1 M HCl. U-87MG glioma cells are implanted into the cranium of a SCID mouse. After 7 days, animals are randomized into three groups of five animals each. One group receives 1 mL i.p. saline injections twice daily for 3 weeks. The two test groups receives 8 mg of sulfasalazine in 1 mL saline twice daily for 3 weeks. Tumor growth and animal health were monitored. After perfusion with 4% paraformaldehyde, mouse brains were collected, rinsed, and placed in 30% sucrose[3]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 689.3±65.0 °C at 760 mmHg |
| Melting Point | 260-265 °C (dec.)(lit.) |
| Molecular Formula | C18H14N4O5S |
| Molecular Weight | 398.393 |
| Flash Point | 370.7±34.3 °C |
| Exact Mass | 398.068481 |
| PSA | 149.69000 |
| LogP | 3.18 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.691 |
| InChIKey | NCEXYHBECQHGNR-UHFFFAOYSA-N |
| SMILES | O=C(O)c1cc(N=Nc2ccc(S(=O)(=O)Nc3ccccn3)cc2)ccc1O |
| Storage condition | Refrigerator |
| Water Solubility | NH4OH 1 M: 50 mg/mL, clear, red | <0.1 g/100 mL at 25 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P333 + P313-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R42/43 |
| Safety Phrases | S22-S29/56-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | VO6250000 |
| HS Code | 2935009090 |
|
~% 
sulfasalazine CAS#:599-79-1 |
| Literature: Przemysl Chemiczny, , vol. 37, p. 162 Chem.Abstr., , p. 13727 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Diplacone and mimulone ameliorate dextran sulfate sodium-induced colitis in rats.
Fitoterapia 101 , 201-7, (2015) Diplacone (1) and mimulone (2), two geranylated flavanones, have previously shown anti-inflammatory and antiradical activity in vitro. The present study aimed to evaluate their activity in vivo on a m... |
|
|
Sulfa drugs inhibit sepiapterin reduction and chemical redox cycling by sepiapterin reductase.
J. Pharmacol. Exp. Ther. 352(3) , 529-40, (2015) Sepiapterin reductase (SPR) catalyzes the reduction of sepiapterin to dihydrobiopterin (BH2), the precursor for tetrahydrobiopterin (BH4), a cofactor critical for nitric oxide biosynthesis and alkylgl... |
|
|
Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: A proof of concept study in healthy subjects.
J. Clin. Pharmacol. 55(1) , 10-6, (2014) During antibiotic treatments, active residuals reaching the colon profoundly affect the bacterial flora resulting in the emergence of resistance. To prevent these effects, we developed an enteric-coat... |
| salazosulfapyridine |
| reupirin |
| sulcolon |
| 2-Hydroxy-5-{[4-(2-pyridinylsulfamoyl)phenyl]diazenyl}benzoic acid |
| Benzoic acid, 2-hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)- |
| salisulf |
| EINECS 209-974-3 |
| Salicylazosulfapyridine |
| 2-Hydroxy-5-{[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl}benzoic acid |
| azopyrin |
| sas-500 |
| benzoic acid, 2-hydroxy-5-[2-[4-[(2-pyridinylamino)sulfonyl]phenyl]diazenyl]- |
| si-88 |
| MFCD00057363 |
| sulfasalazine |
| rorasul |
| accucol |
| benzoic acid, 2-hydroxy-5-[[4-[(2-pyridinylamino)sulfonyl]phenyl]azo]- |
| SSZ |
| sasp |


 CAS#:89-57-6
CAS#:89-57-6
