Matairesinol
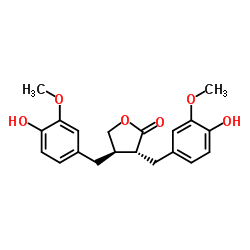
Matairesinol structure
|
Common Name | Matairesinol | ||
|---|---|---|---|---|
| CAS Number | 580-72-3 | Molecular Weight | 358.39 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 593.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C20H22O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 212.3±22.2 °C | |
Use of MatairesinolMatairesinol confers anti-allergic effects in an allergic dermatitis mouse model. DfE-induced changes in IL-4 and IFN-γ mRNA expression in the ears of NC/Nga mice were reversed by matairesinol application[1]. |
| Name | (-)-matairesinol |
|---|---|
| Synonym | More Synonyms |
| Description | Matairesinol confers anti-allergic effects in an allergic dermatitis mouse model. DfE-induced changes in IL-4 and IFN-γ mRNA expression in the ears of NC/Nga mice were reversed by matairesinol application[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 593.0±45.0 °C at 760 mmHg |
| Molecular Formula | C20H22O6 |
| Molecular Weight | 358.39 |
| Flash Point | 212.3±22.2 °C |
| Exact Mass | 358.141632 |
| PSA | 85.22000 |
| LogP | 1.70 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.605 |
| Storage condition | 2-8°C |
|
Optimum yields of dibenzylbutyrolactone-type lignans from Cynareae fruits, during their ripening, germination and enzymatic hydrolysis processes, determined by on-line chromatographic methods.
Phytochem. Anal. 23(6) , 598-603, (2012) Dibenzylbutyrolactone-type lignans are the physiologically active constituents of the achene fruits of Cynareae. These lignans occur in glycoside/aglycone forms: in the highest quantity of the arctiin... |
|
|
Lignans inhibit cell growth via regulation of Wnt/beta-catenin signaling.
Food Chem. Toxicol. 48(8-9) , 2247-52, (2010) As aberrant activation of Wnt/beta-catenin signaling is one of the major mechanisms of carcinogenesis in colon cancer, identification of inhibitors of this pathway may aid in colon cancer prevention. ... |
|
|
Cytotoxic and HIF-1alpha inhibitory compounds from Crossosoma bigelovii.
J. Nat. Prod. 72(5) , 805-12, (2009) Cytotoxicity-guided fractionation of an organic solvent extract of the plant Crossosoma bigelovii led to the discovery of a new strophanthidin glycoside (1) and two new 2-methylchromone glycosides (2 ... |
| (3R,4R)-3,4-Bis(4-hydroxy-3-methoxybenzyl)dihydrofuran-2(3H)-one |
| Matairesinol |
| Matairesil |
| (-)-matairesinol |
| (3R,4R)-3,4-Bis(4-hydroxy-3-methoxybenzyl)dihydro-2(3H)-furanone |
| 2(3H)-Furanone, dihydro-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]-, (3R,4R)- |
| T5OVTJ C1R DQ CO1& D1R DQ CO1 &&(3R,4R)- Form |

