Idarubicin (hydrochloride)
Modify Date: 2024-01-02 15:36:31
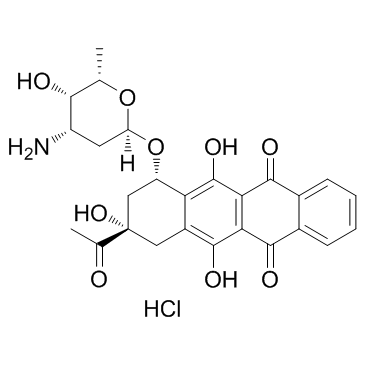
Idarubicin (hydrochloride) structure
|
Common Name | Idarubicin (hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 57852-57-0 | Molecular Weight | 533.955 | |
| Density | N/A | Boiling Point | 725.4ºC at 760 mmHg | |
| Molecular Formula | C26H28ClNO9 | Melting Point | 183-185ºC | |
| MSDS | Chinese USA | Flash Point | 392.5ºC | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of Idarubicin (hydrochloride)Idarubicin hydrochloride is an anthracycline antileukemic drug. It inhibits the topoisomerase II interfering with the replication of DNA and RNA transcription. |
| Name | Idarubicin HCl |
|---|---|
| Synonym | More Synonyms |
| Description | Idarubicin hydrochloride is an anthracycline antileukemic drug. It inhibits the topoisomerase II interfering with the replication of DNA and RNA transcription. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase II |
| In Vitro | The IC50 of idarubicin is 3.3±0.4 ng/mL on MCF-7 monolayers and 7.9±1.1 ng/mL in multicellular spheroids[1]. Idarubicin has shown a greater cytotoxicity than daunorubicin or doxorubicin in various in vitro systems. This has been attributed to a better ability of idarubicin to induce the formation of topoisomerase II -mediated DNA breaks[2].Idarubicin is about 57.5-fold and 25-fold more active than doxorubicin and epirubicin, respectively[3]. Idarubicin produces a concentration-dependent reduction in cell growth, with an IC50 value of approximately 0.01 μM. Idarubicin produced a concentration-dependent inhibition of DNA synthesis[4]. |
| Cell Assay | Stock solutions of idarubicin hydrochloride is dissolved in distilled water (1 mg/mL). MCF-7 monolayer are exposed to idarubicin or its metabolite idarubicinol at 0.01, 0.1, 1, 10, 100, and 1000 ng/mL for 24 hours. Multicellular spheroids are exposed to the same range of idarubicin and idarubicinol concentration as monolayers (0.01-1000 ng/mL) for 24 h and, in separate experiments, at the drug concentration of 100 ng/mL for 6, 12, 24 and 48 h. The inhibition of cell proliferation is determined by counting the viable cells with an hemocytometer. Results are expressed as percentage of cell survival vs. control cultures[1]. |
| References |
[2]. Robert J. Clinical pharmacokinetics of idarubicin. Clin Pharmacokinet. 1993 Apr;24(4):275-88. |
| Boiling Point | 725.4ºC at 760 mmHg |
|---|---|
| Melting Point | 183-185ºC |
| Molecular Formula | C26H28ClNO9 |
| Molecular Weight | 533.955 |
| Flash Point | 392.5ºC |
| Exact Mass | 533.145264 |
| PSA | 176.61000 |
| LogP | 2.52260 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H351-H360 |
| Precautionary Statements | P201-P264-P281-P301 + P310-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+:Verytoxic; |
| Risk Phrases | R60;R61;R28;R40 |
| Safety Phrases | S53-S45 |
| RIDADR | UN 2811 |
| RTECS | HB7877000 |
|
Breakthrough Fusarium solani infection in a patient with acute myeloid leukemia receiving posaconazole prophylaxis.
Ann. Hematol. 93(6) , 1079-81, (2014)
|
|
|
A yeast-based assay identifies drugs that interfere with immune evasion of the Epstein-Barr virus.
Dis. Model Mech. 7(4) , 435-44, (2014) Epstein-Barr virus (EBV) is tightly associated with certain human cancers, but there is as yet no specific treatment against EBV-related diseases. The EBV-encoded EBNA1 protein is essential to maintai... |
|
|
Final report of phase II study of sorafenib, cytarabine and idarubicin for initial therapy in younger patients with acute myeloid leukemia.
Leukemia 28(7) , 1543-5, (2014)
|
| (1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside hydrochloride (1:1) |
| 4-demethoxy-daunomycihydrochloride |
| 4-DMD HCl |
| (1S,3S)-3-Acetyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-6,11-dioxo-1-naphthacenyl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside, hydrochloride |
| 5,12-naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S,9S)-, hydrochloride |
| Idarubicin hcl USP/EP |
| IdarubicinHCl |
| 4-demethoxydaunomycin hydrochloride |
| Idarubicin HCl |
| 5,12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S,9S)-, hydrochloride (1:1) |
| (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside hydrochloride (1:1) |
| (7S-cis)-9-Acetyl-7-((3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxynaphthacene-5,12-dione hydrochloride |
| (1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside hydrochloride (1:1) |
| Idamycin |
| 5,12-naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S,9S)-, hydrochloride (1:1) |
| Zavedos |
| (7S-cis)-9-Acetyl-7-((3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxynaphthacene-5,12-dione Hydrochloride |
| (7S,9S)-9-acetyl-7-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione hydrochloride |
| Idarubicin HCL for research |
| IDARUBICIN HYDROCHLORIDE |
| 4-Demethoxydaunorubicin hydrochloride |
| (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside hydrochloride |
| Idarubicin (hydrochloride) |
 CAS#:60660-74-4
CAS#:60660-74-4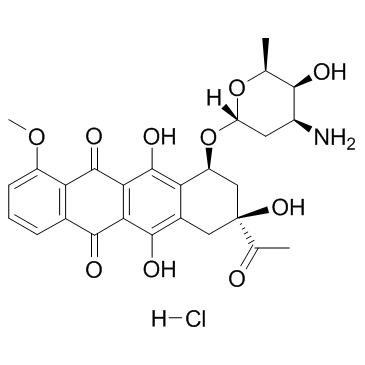 CAS#:23541-50-6
CAS#:23541-50-6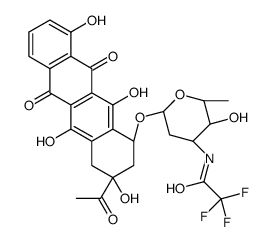 CAS#:68594-06-9
CAS#:68594-06-9![N-[(2S,3S,4S,6R)-6-[[(1S,3S)-3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-2,4-dihydro-1H-tetracen-1-yl]oxy]-3-hydroxy-2-methyloxan-4-yl]-2,2,2-trifluoroacetamide Structure](https://www.chemsrc.com/caspic/421/26388-52-3.png) CAS#:26388-52-3
CAS#:26388-52-3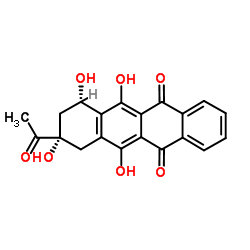 CAS#:60660-75-5
CAS#:60660-75-5![L-lyxo-Hexopyranose,2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-, 1,4-bis(4-nitrobenzoate) (9CI) Structure](https://www.chemsrc.com/caspic/214/52583-22-9.png) CAS#:52583-22-9
CAS#:52583-22-9![5,12-Naphthacenedione,7-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-9-(hydroxyacetyl)-,hydrochloride, (7S,9S)- (9CI) structure](https://www.chemsrc.com/caspic/468/64363-63-9.png) CAS#:64363-63-9
CAS#:64363-63-9
