Hydrocortisone Valerate
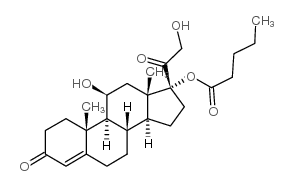
Hydrocortisone Valerate structure
|
Common Name | Hydrocortisone Valerate | ||
|---|---|---|---|---|
| CAS Number | 57524-89-7 | Molecular Weight | 446.57600 | |
| Density | 1.21g/cm3 | Boiling Point | 595.1ºC at 760mmHg | |
| Molecular Formula | C26H38O6 | Melting Point | 217-220 °C | |
| MSDS | Chinese USA | Flash Point | 195ºC | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of Hydrocortisone ValerateHydrocortisone 17-valerate (Cortisol 17-valerate) is a thiazolidine compound[1]. |
| Name | cortisol 17-valerate |
|---|---|
| Synonym | More Synonyms |
| Description | Hydrocortisone 17-valerate (Cortisol 17-valerate) is a thiazolidine compound[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.21g/cm3 |
|---|---|
| Boiling Point | 595.1ºC at 760mmHg |
| Melting Point | 217-220 °C |
| Molecular Formula | C26H38O6 |
| Molecular Weight | 446.57600 |
| Flash Point | 195ºC |
| Exact Mass | 446.26700 |
| PSA | 100.90000 |
| LogP | 3.52270 |
| Index of Refraction | 1.56 |
| InChIKey | FZCHYNWYXKICIO-FZNHGJLXSA-N |
| SMILES | CCCCC(=O)OC1(C(=O)CO)CCC2C3CCC4=CC(=O)CCC4(C)C3C(O)CC21C |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | 40 |
| Safety Phrases | 22-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Topical corticosteroid compounding: effects on physicochemical stability and skin penetration rate.
J. Am. Acad. Dermatol. 21(5 Pt 1) , 979-84, (1989) Compounding compatibility studies of four corticosteroid cream products and four commonly added chemicals are presented. Physical alteration, chemical stability, micropreservative challenge status, an... |
|
|
Addition of a topically applied corticosteroid to a modified Goeckerman regimen for treatment of psoriasis: effect on duration of remission.
J. Am. Acad. Dermatol. 13(5 Pt 1) , 784-91, (1985) A double-blind parallel group study was undertaken to assess the effect of adding a topically applied corticosteroid cream to a modified Goeckerman regimen to treat patients with psoriasis. Nineteen p... |
|
|
Temporal infiltration of leukocyte subsets into mouse skin inflamed with phorbol ester.
Agents Actions 37(3-4) , 260-7, (1992) We have previously shown that multiple topical applications, over 11 days, of the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) induces a persistent inflammatory reaction characterized by e... |
| CORTISOL 17-VALERATE |
| Flexicort |
| Westcort |
| HYDROCORTISONEVALERATE,USP |
| Hydrocortisone Valerate (200 mg) |
| cortisol valerate |
| hydrocortisone valerat |
| hydrocortisone-17-valerate |
| HYDROCORTISONE VALERATE |
 CAS#:600-57-7
CAS#:600-57-7