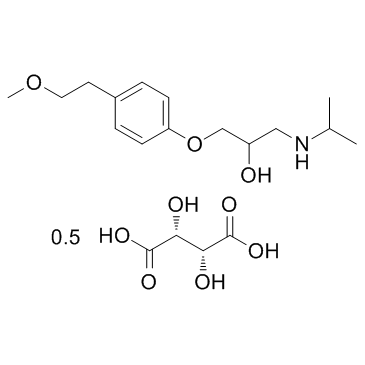
Metoprolol Tartrate
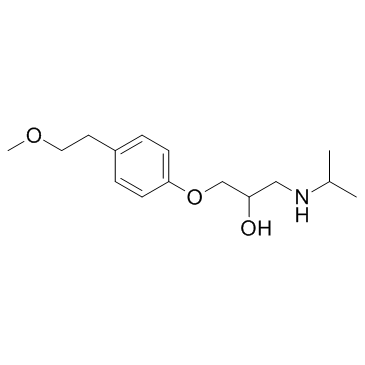
Metoprolol Tartrate structure
|
Common Name | Metoprolol Tartrate | ||
|---|---|---|---|---|
| CAS Number | 56392-17-7 | Molecular Weight | 342.41 | |
| Density | N/A | Boiling Point | 398.6ºC at 760 mmHg | |
| Molecular Formula | C17H28NO6 | Melting Point | 120ºC | |
| MSDS | Chinese USA | Flash Point | 194.9ºC | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of Metoprolol TartrateMetoprolol is a cardioselective β1-adrenergic blocking agent.Target: β1- adrenergic ReceptorPatients took 50 mg metoprolol twice daily with weekly titration to response or 200 mg twice daily. beta(1)-adrenergic receptor polymorphisms are important determinants of antihypertensive response to metoprolol. In the future, codon 49 and 389 genotypes or beta(1)-adrenergic receptor haplotypes might be used to predict the diastolic blood pressure response to metoprolol in patients with hypertension [1]. Patients were studied at baseline and after each dose titration of metoprolol succinate (at 25, 50, 100, and 200 mg once/day) and immediate-release carvedilol (at 3.125, 6.25, 12.5, and 25 mg twice/day). As assessed by glucose AUC, there was no significant difference in the degree of beta(2)-blockade between metoprolol 200 mg and carvedilol 25 mg. In contrast to these data, the degree of beta(2)-blockade as assessed by potassium AUC was greater for carvedilol compared with metoprolol across all doses [2]. |
| Name | rac Metoprolol Hemi (+)-Tartrate |
|---|---|
| Synonym | More Synonyms |
| Description | Metoprolol is a cardioselective β1-adrenergic blocking agent.Target: β1- adrenergic ReceptorPatients took 50 mg metoprolol twice daily with weekly titration to response or 200 mg twice daily. beta(1)-adrenergic receptor polymorphisms are important determinants of antihypertensive response to metoprolol. In the future, codon 49 and 389 genotypes or beta(1)-adrenergic receptor haplotypes might be used to predict the diastolic blood pressure response to metoprolol in patients with hypertension [1]. Patients were studied at baseline and after each dose titration of metoprolol succinate (at 25, 50, 100, and 200 mg once/day) and immediate-release carvedilol (at 3.125, 6.25, 12.5, and 25 mg twice/day). As assessed by glucose AUC, there was no significant difference in the degree of beta(2)-blockade between metoprolol 200 mg and carvedilol 25 mg. In contrast to these data, the degree of beta(2)-blockade as assessed by potassium AUC was greater for carvedilol compared with metoprolol across all doses [2]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 398.6ºC at 760 mmHg |
|---|---|
| Melting Point | 120ºC |
| Molecular Formula | C17H28NO6 |
| Molecular Weight | 342.41 |
| Flash Point | 194.9ºC |
| PSA | 216.50000 |
| LogP | 1.88560 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25 |
| Safety Phrases | 7-16-36/37-45 |
| RIDADR | NONH for all modes of transport |
| RTECS | UB7450100 |
|
~72% 
Metoprolol Tartrate CAS#:56392-17-7 |
| Literature: IPCA LABORATORIES LIMITED Patent: WO2005/46568 A2, 2005 ; Location in patent: Page/Page column 9-10 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU.
J. Chromatogr. A. 1418 , 140-9, (2015) The fate and removal of organic micropollutants in the environment is a demanding issue evidenced by the recent European policy. This work presents an analytical method for the trace quantification of... |
|
|
Human and simulated intestinal fluids as solvent systems to explore food effects on intestinal solubility and permeability.
Eur. J. Pharm. Sci. 63 , 178-86, (2014) The mixed micelles and vesicles present in the intraluminal environment of the postprandial state exhibit suitable solubilizing capacities for lipophilic drugs. This increase in solubility, however, i... |
|
|
Evaluation of the effect of TM208 on the activity of five cytochrome P450 enzymes using on-line solid-phase extraction HPLC-DAD: a cocktail approach.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 923-924 , 29-36, (2013) A rapid, simple, and sensitive on-line solid-phase extraction HPLC-DAD method for simultaneous evaluation of the activity of five CYP450 isoforms (CYP1A2, CYP2C19, CYP2D6, CYP2E1 and CYP3A4) in vivo h... |
| UNII:W5S57Y3A5L |
| Metoprolol tartrate |
| MFCD00941460 |
| (2R,3R)-2,3-Dihydroxysuccinic acid - 1-(isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol (1:2) |
| Butanedioic acid, 2,3-dihydroxy-, (2R,3R)-, compd. with 1-[4-(2-methoxyethyl)phenoxy]-3-[(1-methylethyl)amino]-2-propanol (1:2) |
| (2R,3R)-2,3-Dihydroxysuccinic acid - 1-(isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]-2-propanol (1:2) |
| EINECS 260-148-9 |
| (±)-Metoprolol (+)-tartrate salt |
| Metoprolol (Tartrate) |