Epirubicin hydrochloride
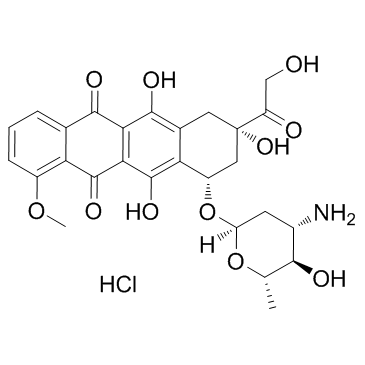
Epirubicin hydrochloride structure
|
Common Name | Epirubicin hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 56390-09-1 | Molecular Weight | 579.980 | |
| Density | 1.61g/cm3 | Boiling Point | 810.3ºC at 760 mmHg | |
| Molecular Formula | C27H30ClNO11 | Melting Point | 185ºC dec | |
| MSDS | N/A | Flash Point | 443.8ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Epirubicin hydrochlorideEpirubicin (hydrochloride) is a semisynthetic L-arabino derivative of doxorubicin, and an antineoplastic agent by inhibiting Topoisomerase. |
| Name | Epirubicin hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Epirubicin (hydrochloride) is a semisynthetic L-arabino derivative of doxorubicin, and an antineoplastic agent by inhibiting Topoisomerase. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase |
| In Vitro | Epirubicin, like doxorubicin, exerts its antitumor effects by complex with DNA, resulting in damage to DNA and interference with the synthesis of DNA, RNA, and proteins. Epirubicin may also affect the integrity and activity of cellular membranes. Maximal cell kill caused by Epirubicin occurs during the S phase of the cell cycle. With higher concentrations effects are also seen in early G2 as well as G1 and M phases[1]. Epirubicin display antineoplastic activity against most cancer cells. Epirubicin is cytotoxic to Hepatoma G2 cells with IC50 of 1.6 μg/mL at 24 hr. 1.6 μg/mL Epirubicin induces apoptosis of Hep G2 cells, and higher activity of catalase by 50%, Se-dependent glutathione peroxidase by 110%, Cu, Zn-superoxide dismutase by 172% and Mn-superoxide dismutase by 135%. Epirbicin increases the cellular expression of NADPH-CYP 450 reductase, and reduces GST-π expression[2]. |
| In Vivo | Epirubicin are clinically active against a broad range of tumor types, including breast cancer, malignant lymphomas, soft tissue sarcomas, lung cancer, pleural mesothelioma, gastrointestinal cancer, head and neck cancer, ovarian cancer, prostatic carcinoma, transitional bladder carcinoma and so on[3]. Epirubicin at a dose of 3.5 mg/kg suppresses tumor mass of human breast tumor xenograft R-27 by 74.4 %[4]. |
| Cell Assay | Hep G2 cells (500 cells/well, monolayer) are plated in a 96-well plate. The next day the cells are treated with Epirubicin in the medium. At the end of the incubation periods, 15% volume of MTT dye solution is added. After 1 hr of incubation at 37°C, an equal volume of solubilization/stop solution (dimethylsul-foxide) is added to each well for an additional 1 hr incubation. The absorbance of the reaction solution at 570 nm is recorded. |
| References |
[3]. Bonadonna, G., et al. Drugs ten years later: epirubicin. Ann Oncol, 1993. 4(5): p. 359-69. |
| Density | 1.61g/cm3 |
|---|---|
| Boiling Point | 810.3ºC at 760 mmHg |
| Melting Point | 185ºC dec |
| Molecular Formula | C27H30ClNO11 |
| Molecular Weight | 579.980 |
| Flash Point | 443.8ºC |
| Exact Mass | 579.150757 |
| PSA | 206.07000 |
| LogP | 1.50360 |
| Index of Refraction | 1.709 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H340-H350-H360 |
| Precautionary Statements | P201-P280-P301 + P312 + P330-P308 + P313 |
| Hazard Codes | Xn |
| Risk Phrases | 22-63-62-46-40 |
| Safety Phrases | 36/37/38-45-46 |
| RIDADR | NONH for all modes of transport |
| RTECS | QI9295750 |
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Fourier transform infrared spectroscopy imaging of live epithelial cancer cells under non-aqueous media.
J. Clin. Pathol. 66(4) , 312-8, (2013) Fourier transform infrared (FT-IR) imaging is increasingly being applied to biomedical specimens, but strong IR absorption by water complicates live cell imaging. This study investigates the viability... |
|
|
Intraabdominal follicular dendritic cell sarcoma: a report of three cases and review of the literature.
Tumori 99(2) , e65-9, (2013) Follicular dendritic cell sarcoma (FDCS) is a rare neoplasm that originates from follicular dendritic cells in lymphoid follicles. FDCS has been increasingly reported in recent years. However, data on... |
|
|
Treatment outcome in children and adolescents with relapsed Hodgkin lymphoma--results of the UK HD3 relapse treatment strategy.
Br. J. Haematol. 165(4) , 534-44, (2014) The purpose of this national retrospective study was to evaluate the outcome in children with relapsed or primary refractory Hodgkin lymphoma [HL] after a primary chemotherapy alone treatment strategy... |
| 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)-, hydrochloride |
| (1S,3S)-3-Glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside hydrochloride (1:1) |
| 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-.α.-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, hydrochloride |
| Pidorubicin hydrochloride |
| 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)-, hydrochloride (1:1) |
| Ellence |
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside hydrochloride |
| Epirubicin hydrochloride |
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside hydrochloride (1:1) |
| EINECS 260-145-2 |
| (8S,10S)-10-{[(2R,4S,5R,6S)-4-Amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracen-5,12-dionhydrochlorid |
| EPIRUBICIN HCl |
| (8S,10S)-10-{[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione hydrochloride |
| Epirubicin (hydrochloride) |

