Beclomethasone 17-Propionate
Modify Date: 2025-08-24 22:56:40
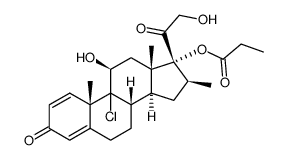
Beclomethasone 17-Propionate structure
|
Common Name | Beclomethasone 17-Propionate | ||
|---|---|---|---|---|
| CAS Number | 5534-18-9 | Molecular Weight | 464.97900 | |
| Density | 1.3g/cm3 | Boiling Point | 608.9ºC at 760mmHg | |
| Molecular Formula | C25H33ClO6 | Melting Point | 124-126ºC | |
| MSDS | N/A | Flash Point | 322ºC | |
Use of Beclomethasone 17-PropionateBeclomethasone 17-propionate (Beclomethasone-17-monopropionate), an active metabolite of Beclomethasone dipropionate (HY-13571), is a glucocorticoid receptor (GR) agonist. Beclomethasone 17-propionate exhibits greater affinity for GR than Beclomethasone dipropionate. Beclomethasone 17-propionate effectively suppresses cytokine production in chronic obstructive pulmonary disease (COPD) lung macrophages[1][2][3]. |
| Name | Beclomethasone 17-Propionate |
|---|---|
| Synonym | More Synonyms |
| Description | Beclomethasone 17-propionate (Beclomethasone-17-monopropionate), an active metabolite of Beclomethasone dipropionate (HY-13571), is a glucocorticoid receptor (GR) agonist. Beclomethasone 17-propionate exhibits greater affinity for GR than Beclomethasone dipropionate. Beclomethasone 17-propionate effectively suppresses cytokine production in chronic obstructive pulmonary disease (COPD) lung macrophages[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Metabolism of Beclomethasone dipropionate to 17-BMP is an important activation step. Beclomethasone 17-propionate inhibits LPS-stimulated CXCL8, TNFα and IL-6. The EC50 values of Beclomethasone 17-propionate for IL-6, TNFα and CXCL8 were 0.05 nM, 0.01 nM and 0.1 nM, respectively. Beclomethasone 17-propionate evokes upregulation of the GR dependent genes FKBP51 and GILZ[3]. |
| References |
| Density | 1.3g/cm3 |
|---|---|
| Boiling Point | 608.9ºC at 760mmHg |
| Melting Point | 124-126ºC |
| Molecular Formula | C25H33ClO6 |
| Molecular Weight | 464.97900 |
| Flash Point | 322ºC |
| Exact Mass | 464.19700 |
| PSA | 100.90000 |
| LogP | 3.12590 |
| Index of Refraction | 1.583 |
| Storage condition | -20°C |
| [(8S,9R,10S,11S,13S,14S,16S,17R)-9-chloro-11-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate |