Amitriptyline Hydrochloride

Amitriptyline Hydrochloride structure
|
Common Name | Amitriptyline Hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 549-18-8 | Molecular Weight | 313.864 | |
| Density | 1.076g/cm3 | Boiling Point | 398.2ºC at 760 mmHg | |
| Molecular Formula | C20H24ClN | Melting Point | 196-197°C | |
| MSDS | Chinese USA | Flash Point | 11 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of Amitriptyline HydrochlorideAmitriptyline Hydrochloride is a dibenzocycloheptene-derivative tricyclic antidepressant (TCA).Target: OthersAmitriptyline acts primarily as a serotonin-norepinephrine reuptake inhibitor, with strong actions on the serotonin transporter and moderate effects on the norepinephrine transporter. It has negligible influence on the dopamine transporter and therefore does not affect dopamine reuptake, being nearly 1,000 times weaker on it than on serotonin [1]. Amitriptyline additionally functions as a 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, 5-HT7, α1-adrenergic, H1, H2, and mACh receptorantagonist, and σ1 receptor agonist. It has also been shown to be a relatively weak NMDA receptor negative allosteric modulator at the same binding site as phencyclidine. Amitriptyline inhibits sodium channels, L-type calcium channels, and Kv1.1, Kv7.2, and Kv7.3 voltage-gated potassium channels, and therefore acts as a sodium, calcium, and potassium channel blocker as well [2]. Recently, amitriptyline has been demonstrated to act as an agonist of the TrkA and TrkB receptors. It promotes the heterodimerization of these proteins in the absence of NGF and has potent neurotrophic activity both in-vivo and in-vitro in mouse models [3]. |
| Name | Amitriptyline hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Amitriptyline Hydrochloride is a dibenzocycloheptene-derivative tricyclic antidepressant (TCA).Target: OthersAmitriptyline acts primarily as a serotonin-norepinephrine reuptake inhibitor, with strong actions on the serotonin transporter and moderate effects on the norepinephrine transporter. It has negligible influence on the dopamine transporter and therefore does not affect dopamine reuptake, being nearly 1,000 times weaker on it than on serotonin [1]. Amitriptyline additionally functions as a 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, 5-HT7, α1-adrenergic, H1, H2, and mACh receptorantagonist, and σ1 receptor agonist. It has also been shown to be a relatively weak NMDA receptor negative allosteric modulator at the same binding site as phencyclidine. Amitriptyline inhibits sodium channels, L-type calcium channels, and Kv1.1, Kv7.2, and Kv7.3 voltage-gated potassium channels, and therefore acts as a sodium, calcium, and potassium channel blocker as well [2]. Recently, amitriptyline has been demonstrated to act as an agonist of the TrkA and TrkB receptors. It promotes the heterodimerization of these proteins in the absence of NGF and has potent neurotrophic activity both in-vivo and in-vitro in mouse models [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.076g/cm3 |
|---|---|
| Boiling Point | 398.2ºC at 760 mmHg |
| Melting Point | 196-197°C |
| Molecular Formula | C20H24ClN |
| Molecular Weight | 313.864 |
| Flash Point | 11 °C |
| Exact Mass | 313.159729 |
| PSA | 3.24000 |
| LogP | 4.97060 |
| InChIKey | KFYRPLNVJVHZGT-UHFFFAOYSA-N |
| SMILES | CN(C)CCC=C1c2ccccc2CCc2ccccc21.Cl |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R23/24/25;R36/37/38;R42/43;R63 |
| Safety Phrases | S22-S26-S36/37/39-S45-S36/37-S16 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | HO9450000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
Antidepressants activate the lysophosphatidic acid receptor LPA(1) to induce insulin-like growth factor-I receptor transactivation, stimulation of ERK1/2 signaling and cell proliferation in CHO-K1 fibroblasts.
Biochem. Pharmacol. 95 , 311-23, (2015) Different lines of evidence indicate that the lysophosphatidic acid (LPA) receptor LPA1 is involved in neurogenesis, synaptic plasticity and anxiety-related behavior, but little is known on whether th... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predicti... |
| Amitriptyline Hydrochloride |
| 3-(5,6-dihydrodibenzo[2,1-b:2',1'-f][7]annulen-11-ylidene)-N,N-dimethylpropan-1-amine,hydrochloride |
| 3-(10,11-Dihydro-5H-dibenzo[a,d][7]annulen-5-yliden)-N,N-dimethylpropan-1-aminhydrochlorid |
| 3-(10,11-Dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N,N-dimethylpropan-1-amine hydrochloride (1:1) |
| EINECS 208-964-6 |
| 1-Propanamine, 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-, hydrochloride (1:1) |
| 1-propanamine, 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-, hydrochloride |
| MFCD00012537 |
| Amitriptyline HCl |
| 3-(10,11-dihydro-5H-dibenzo[a,d][7]annulén-5-ylidène)-N,N-diméthylpropan-1-amine chlorhydrate |
| 3-(10,11-Dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride (1:1) |
| 3-(10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N,N-dimethylpropan-1-amine hydrochloride |
| 5-(3-Dimethylaminopropylidene)dibenzo[a,d][1,4]cycloheptadiene hydrochloride |
| 10,11-Dihydro-N,N-dimethyl-5H-dibenzo[a,d]cycloheptene-δ5,γ-propylamine hydrochloride |
| Amitriptyline (hydrochloride) |
![5-(3-dimethylaminopropyl)-10,11-dihydrodibenzo[a,d]cyclohepten-5-ol Structure](https://image.chemsrc.com/caspic/040/1159-03-1.png) CAS#:1159-03-1
CAS#:1159-03-1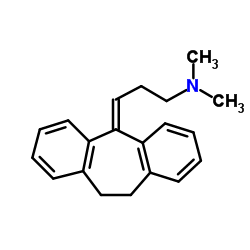 CAS#:50-48-6
CAS#:50-48-6 CAS#:1210-35-1
CAS#:1210-35-1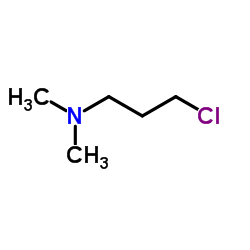 CAS#:109-54-6
CAS#:109-54-6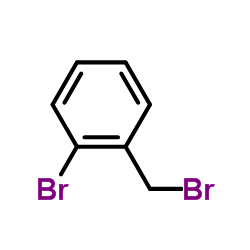 CAS#:3433-80-5
CAS#:3433-80-5
