QX-222 chloride
Modify Date: 2025-08-26 23:52:36
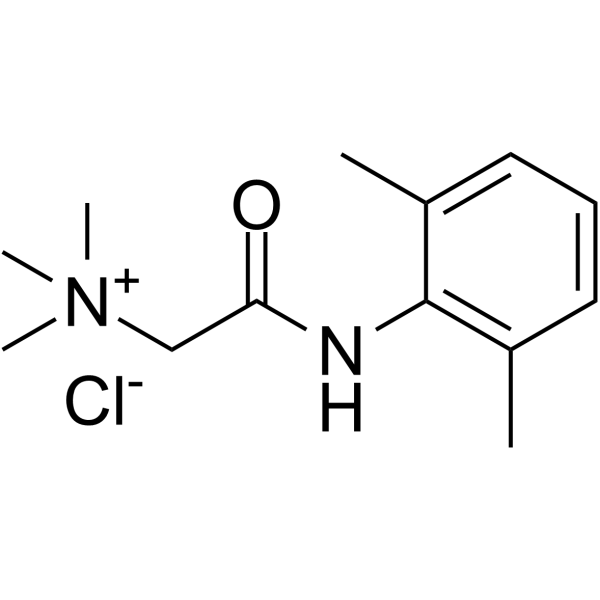
QX-222 chloride structure
|
Common Name | QX-222 chloride | ||
|---|---|---|---|---|
| CAS Number | 5369-00-6 | Molecular Weight | 256.77200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C13H21ClN2O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of QX-222 chlorideQX-222 chloride, a trimethyl analogue of Lignocaine (HY-B0185), is a potent Na+ channel blocker[1][2][3]. |
| Name | 2-[(2,6-Dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethanaminium c hloride |
|---|
| Description | QX-222 chloride, a trimethyl analogue of Lignocaine (HY-B0185), is a potent Na+ channel blocker[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Twelve minutes after external application of 500 μM QX222 chloride, μ1 IP-Loop to Heart Sequence (μ1-Y401C) results in a significant block compared with μ1-WT (WT, 14.2±1.6% block, n = 8; Y401C, 45.2±3.6% block, n = 9; P < 0.001)[1]. |
| In Vivo | QX-222 (10 mg/kg; intravenous infusion 7 days) chloride reverses spinal nerve ligation (SNL)-induced thermal hypersensitivity and induced antinociception in sham-operated rats[2]. |
| References |
| Molecular Formula | C13H21ClN2O |
|---|---|
| Molecular Weight | 256.77200 |
| Exact Mass | 256.13400 |
| PSA | 35.42000 |
| LogP | 3.27210 |
| InChIKey | WFKXSWWTOZBDME-UHFFFAOYSA-N |
| SMILES | Cc1cccc(C)c1NC(=O)C[N+](C)(C)C.[Cl-] |
| Hazard Codes | Xi |
|---|