4-Isopropylbenzoic acid
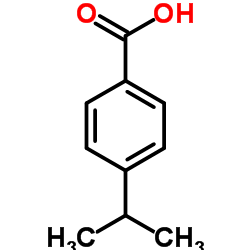
4-Isopropylbenzoic acid structure
|
Common Name | 4-Isopropylbenzoic acid | ||
|---|---|---|---|---|
| CAS Number | 536-66-3 | Molecular Weight | 164.201 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 271.8±19.0 °C at 760 mmHg | |
| Molecular Formula | C10H12O2 | Melting Point | 117-120 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 128.1±16.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-Isopropylbenzoic acid4-Isopropylbenzoic acid, an aromatic monoterpenoid, is isolated from the stem bark of Bridelia retusa. 4-Isopropylbenzoic acid exhibits antifungal activities. 4-Isopropylbenzoic acid is also a reversible and uncompetitive inhibitor of mushroom tyrosinase[1][2]. |
| Name | p-cumic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Isopropylbenzoic acid, an aromatic monoterpenoid, is isolated from the stem bark of Bridelia retusa. 4-Isopropylbenzoic acid exhibits antifungal activities. 4-Isopropylbenzoic acid is also a reversible and uncompetitive inhibitor of mushroom tyrosinase[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 271.8±19.0 °C at 760 mmHg |
| Melting Point | 117-120 °C(lit.) |
| Molecular Formula | C10H12O2 |
| Molecular Weight | 164.201 |
| Flash Point | 128.1±16.2 °C |
| Exact Mass | 164.083725 |
| PSA | 37.30000 |
| LogP | 3.23 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.535 |
| Water Solubility | sparingly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DH3850500 |
| HS Code | 2916399090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916399090 |
|---|---|
| Summary | 2916399090 other aromatic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors.
Bioorg. Med. Chem. 15 , 2006-15, (2007) Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-q... |
|
|
Anaerobic activation of p-cymene in denitrifying betaproteobacteria: methyl group hydroxylation versus addition to fumarate.
Appl. Environ. Microbiol. 80(24) , 7592-603, (2014) The betaproteobacteria "Aromatoleum aromaticum" pCyN1 and "Thauera" sp. strain pCyN2 anaerobically degrade the plant-derived aromatic hydrocarbon p-cymene (4-isopropyltoluene) under nitrate-reducing c... |
|
|
Bacterial metabolism of side chain fluorinated aromatics: cometabolism of 4-trifluoromethyl(TFM)-benzoate by 4-isopropylbenzoate grown Pseudomonas putida JT strains.
Arch. Microbiol. 149(3) , 198-206, (1988) Enzymes of the p-cymene pathway in Pseudomonas putida strains cometabolized the intermediate analogue 4-trifluoromethyl(TFM)benzoate. Three products, 4-TFM-2,3-dihydro-2,3-dihydroxybenzoate, 4-TFM-2,3... |
| p-cumic acid |
| EINECS 208-642-5 |
| cumic acid |
| Benzoic acid, 4-(1-methylethyl)- |
| 4-propan-2-ylbenzoic acid |
| 4-Isopropylbenzoic acid |
| p-Isopropylbenzoic acid |
| CuMinic Acid |
| MFCD00002564 |
 CAS#:122-03-2
CAS#:122-03-2 CAS#:536-60-7
CAS#:536-60-7 CAS#:99-87-6
CAS#:99-87-6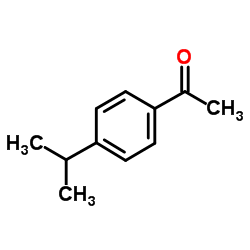 CAS#:645-13-6
CAS#:645-13-6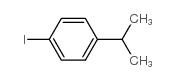 CAS#:17356-09-1
CAS#:17356-09-1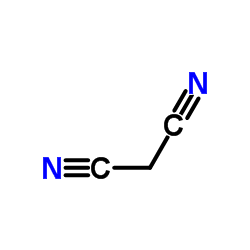 CAS#:109-77-3
CAS#:109-77-3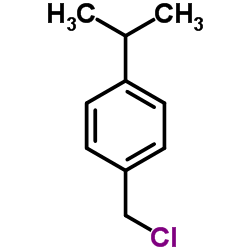 CAS#:2051-18-5
CAS#:2051-18-5 CAS#:20185-55-1
CAS#:20185-55-1 CAS#:75-30-9
CAS#:75-30-9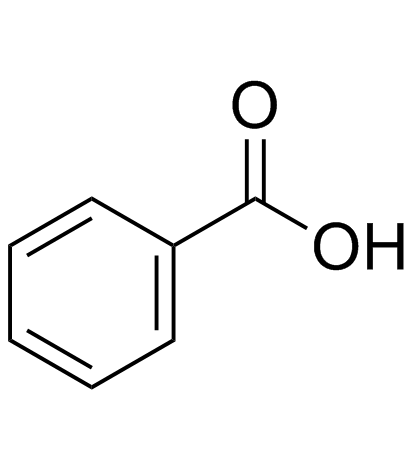 CAS#:65-85-0
CAS#:65-85-0 CAS#:21900-62-9
CAS#:21900-62-9 CAS#:19024-50-1
CAS#:19024-50-1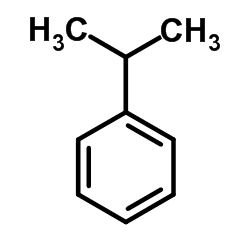 CAS#:98-82-8
CAS#:98-82-8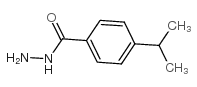 CAS#:5351-24-6
CAS#:5351-24-6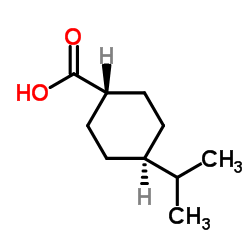 CAS#:7077-05-6
CAS#:7077-05-6 CAS#:7084-93-7
CAS#:7084-93-7 CAS#:13816-33-6
CAS#:13816-33-6 CAS#:619-76-1
CAS#:619-76-1 CAS#:3609-50-5
CAS#:3609-50-5
