N-Hydroxyphthalimide

N-Hydroxyphthalimide structure
|
Common Name | N-Hydroxyphthalimide | ||
|---|---|---|---|---|
| CAS Number | 524-38-9 | Molecular Weight | 163.130 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 370.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H5NO3 | Melting Point | 233 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 177.8±23.2 °C | |
| Name | N-Hydroxyphthalimide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 370.3±25.0 °C at 760 mmHg |
| Melting Point | 233 °C (dec.)(lit.) |
| Molecular Formula | C8H5NO3 |
| Molecular Weight | 163.130 |
| Flash Point | 177.8±23.2 °C |
| Exact Mass | 163.026947 |
| PSA | 57.61000 |
| LogP | 0.42 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.718 |
| InChIKey | CFMZSMGAMPBRBE-UHFFFAOYSA-N |
| SMILES | O=C1c2ccccc2C(=O)N1O |
| Storage condition | Store at RT. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TI5200000 |
| HS Code | 29251995 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2925190090 |
|---|---|
| Summary | 2925190090 other imides and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Bromide-free oxidizing system for carboxylic moiety formation in cellulose chain.
Carbohydr. Polym. 90(4) , 1415-9, (2012) NHPI (N-hydroxyphthalimide) was used to mediate the oxidation of cellulose fibers in the absence of sodium bromide, as traditionally was used in this kind of transformations, solely using sodium hypoc... |
|
|
Anthrone-derived NHPI analogues as catalysts in reactions using oxygen as an oxidant.
Org. Biomol. Chem. 6(22) , 4096-8, (2008) An enantioselective synthesis of anthrone-derived NHPI analogues has been developed. One of these analogues, in combination with Co salts, was employed to catalyse the aerobic oxidation of benzylic co... |
|
|
Oxidized cellulose--survey of the most recent achievements.
Carbohydr. Polym. 93(1) , 207-15, (2013) The functionalization and particularly the oxidation of cellulose is an intriguing and challenging topic due to the presence of multiple reactive sites, which can undergo specific reactions. The varie... |
| nhpi |
| N-Hydroxy Phthalimide |
| 2-Hydroxy-1H-isoindole-1,3(2H)-dione |
| 2-hydroxyisoindoline-1,3-dione |
| N-Hydroxyphthalimide |
| HOPHT |
| EINECS 208-358-1 |
| N-hydroxylphthalimide |
| phthalamohydroxamic acid |
| PHTHALOXIME |
| N-hydroxyphtalimide |
| n-hydroxy-phthalimid |
| MFCD00005891 |
| hydroxyphthalimide |
| NHPI NOP |
| 1H-Isoindole-1,3(2H)-dione, 2-hydroxy- |
| N-hydroxy-phthalimide |
| 2-hydroxy-1,3-isoindolinedione |
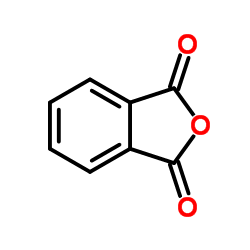 CAS#:85-44-9
CAS#:85-44-9 CAS#:150220-29-4
CAS#:150220-29-4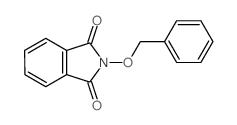 CAS#:16653-19-3
CAS#:16653-19-3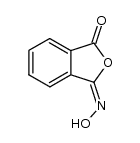 CAS#:57685-30-0
CAS#:57685-30-0 CAS#:84-66-2
CAS#:84-66-2![Benzoic acid,2-[(hydroxyimino)methyl]- Structure](https://image.chemsrc.com/caspic/049/6383-59-1.png) CAS#:6383-59-1
CAS#:6383-59-1 CAS#:71-43-2
CAS#:71-43-2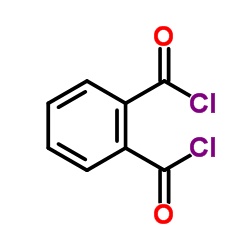 CAS#:88-95-9
CAS#:88-95-9 CAS#:4727-17-7
CAS#:4727-17-7 CAS#:38945-21-0
CAS#:38945-21-0 CAS#:39020-79-6
CAS#:39020-79-6 CAS#:4616-63-1
CAS#:4616-63-1 CAS#:93-89-0
CAS#:93-89-0 CAS#:142232-07-3
CAS#:142232-07-3 CAS#:39917-38-9
CAS#:39917-38-9 CAS#:38228-99-8
CAS#:38228-99-8 CAS#:1724-39-6
CAS#:1724-39-6 CAS#:2142-03-2
CAS#:2142-03-2
