Mezlocillin
Modify Date: 2025-08-21 20:03:25
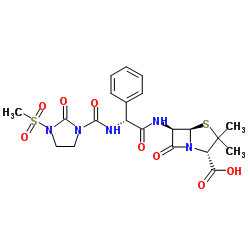
Mezlocillin structure
|
Common Name | Mezlocillin | ||
|---|---|---|---|---|
| CAS Number | 51481-65-3 | Molecular Weight | 539.582 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 655.5ºC at 760 mmHg | |
| Molecular Formula | C21H25N5O8S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 350.2ºC | |
Use of MezlocillinMezlocillin (BAY-f 1353) is a β-lactam antibiotic, a semisynthetic and extended-spectrum antibiotic. Mezlocillin is active against both gram-negative and gram-positive bacteria. Mezlocillin can be used in bacterial infection research[1][2]. |
| Name | mezlocillin |
|---|---|
| Synonym | More Synonyms |
| Description | Mezlocillin (BAY-f 1353) is a β-lactam antibiotic, a semisynthetic and extended-spectrum antibiotic. Mezlocillin is active against both gram-negative and gram-positive bacteria. Mezlocillin can be used in bacterial infection research[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Mezlocillin (subcutaneous injection; 1.7-5.7 mg/kg; twice daily; 7 d) treatment in vivo suppresses the IgM and IgG responses and the delayed-type hypersensitivity reaction, observes the loss of hair, and inhibits lymphocyte proliferation of animals treated with all doses[1]. Animal Model: Male Balb/c mice[1] Dosage: 1.7, 4.3, and 5.7 mg/kg Administration: Subcutaneous injection; 1.7, 4.3, and 5.7 mg/kg; twice daily; 7 days Result: Suppressed the IgM and IgG responses and the delayed-type hypersensitivity reaction treated with all doses. Observed a loss of hair in the majority of animals treated with all doses. Inhibited lymphocyte proliferation of spleen-cell cultures from animals. |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 655.5ºC at 760 mmHg |
| Molecular Formula | C21H25N5O8S2 |
| Molecular Weight | 539.582 |
| Flash Point | 350.2ºC |
| Exact Mass | 539.114441 |
| PSA | 207.18000 |
| LogP | -1.18 |
| Index of Refraction | 1.701 |
|
~90% 
Mezlocillin CAS#:51481-65-3 |
| Literature: Koenig, H.-B.; Metzger, K. G.; Offe, H.-A.; Schroeck, W. Arzneimittel Forschung, 1983 , vol. 33, # 1 p. 88 - 90 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| 6R-[2-[3-(Methylsulfonyl)-2-oxo-1-imidazolidine Carboxamido]-2-phenylacetamido]penicillanic Acid |
| EINECS 257-233-8 |
| (2S,5R,6R)-3,3-Dimethyl-6-{[(2R)-2-({[3-(methylsulfonyl)-2-oxoimidazolidin-1-yl]carbonyl}amino)-2-phenylacetyl]amino}-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| [2S-[2a,5a,6b(S*)]]-3,3-Dimethyl-6-[[[[[3-(methylsulfonyl)-2-oxo-1-imidazolidinyl]carbonyl]amino]phenylacetyl]amino]-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-6-[[(2R)-2-[[[3-(methylsulfonyl)-2-oxo-1-imidazolidinyl]carbonyl]amino]-2-phenylacetyl]amino]-7-oxo-, (2S,5R,6R)- |
| (2S,5R,6R)-3,3-Dimethyl-6-((R)-2-(3-(methylsulfonyl)-2-oxo-1-imidazolidinecarboxamido)-2-phenylacetamido)-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid |
| Antibiotic bay-f 1353 |
| Mezlocillin |
| Meloxacam acid |
| MFCD00056866 |
| Melocillin sodium |
| antibioticbay-f1353 |
| Multocillin |
| Bay f 1353 |
| (2S,5R,6R)-3,3-Dimethyl-6-{[(2R)-2-({[3-(methylsulfonyl)-2-oxo-1-imidazolidinyl]carbonyl}amino)-2-phenylacetyl]amino}-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| (2S,5R,6R)-3,3-Dimethyl-6-((R)-2-(3-(methylsulfonyl)-2-oxo-1-imidazolidinecarboxamido)-2-phenylacetamido)-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid |

