Ro 4468
Modify Date: 2025-08-25 22:08:18
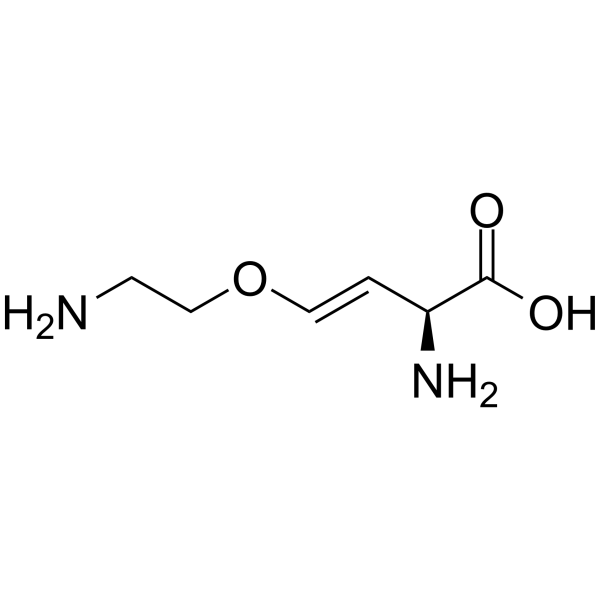
Ro 4468 structure
|
Common Name | Ro 4468 | ||
|---|---|---|---|---|
| CAS Number | 49669-74-1 | Molecular Weight | 160.17100 | |
| Density | 1.232g/cm3 | Boiling Point | 363.7ºC at 760 mmHg | |
| Molecular Formula | C6H12N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 173.7ºC | |
Use of Ro 4468Aviglycine (ABG-3168 free base) is an inhibitor of ethylene biosynthesis. Aviglycine (ABG-3168 (free base)) application delays natural flowering in pineapple[1]. |
| Name | aviglycine |
|---|---|
| Synonym | More Synonyms |
| Description | Aviglycine (ABG-3168 free base) is an inhibitor of ethylene biosynthesis. Aviglycine (ABG-3168 (free base)) application delays natural flowering in pineapple[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.232g/cm3 |
|---|---|
| Boiling Point | 363.7ºC at 760 mmHg |
| Molecular Formula | C6H12N2O3 |
| Molecular Weight | 160.17100 |
| Flash Point | 173.7ºC |
| Exact Mass | 160.08500 |
| PSA | 98.57000 |
| LogP | 0.28790 |
| Index of Refraction | 1.531 |
| InChIKey | USGUVNUTPWXWBA-JRIXXDKMSA-N |
| SMILES | NCCOC=CC(N)C(=O)O |
|
Name: Human Cystathionine gamma-lyase (Hydrogen sulphide synthesis)
Source: IUPHAR-DB
Target: Cystathionine gamma-lyase (Hydrogen sulphide synthesis) [Homo sapiens]
External Id: 1444_Human
|
|
Name: Inhibition of cystathionine gamma-lyase (unknown origin)
Source: ChEMBL
Target: Cystathionine gamma-lyase
External Id: CHEMBL4136611
|
Total 2, Current Page 1 of 1
1
| Ro-4468 |
| (2S,3E)-2-Amino-4-(2-aminoethoxy)-3-butenoic acid |
| TMP-941 |
| Aviglycine |
| (2S,3E)-2-amino-4-(2-aminoethoxy)but-3-enoic acid |
| Ro 20-4468/001 |
| ANTIBIOTIC RO 20-4468/001 |
| AVG |
| (2S,3E)-2-amino-4-(2-aminoethoxy)-3-butenoic acid |
| (E)-L-2-[2-(2-aminoethoxy)vinyl]glycine |
| [S-(E)]-2-amino-4-(2-aminoethoxy)-3-butenoic acid |