epi-Inositol
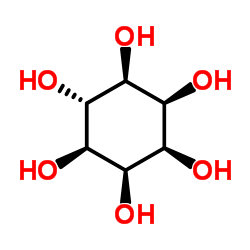
epi-Inositol structure
|
Common Name | epi-Inositol | ||
|---|---|---|---|---|
| CAS Number | 488-58-4 | Molecular Weight | 180.156 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 291.3±40.0 °C at 760 mmHg | |
| Molecular Formula | C6H12O6 | Melting Point | 304°C(dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 143.4±21.9 °C | |
Use of epi-Inositolepi-Inositol is an endogenous metabolite present in Cerebrospinal_Fluid that can be used for the research of Ethanol Consumption[1][2]. |
| Name | epi-Inositol |
|---|---|
| Synonym | More Synonyms |
| Description | epi-Inositol is an endogenous metabolite present in Cerebrospinal_Fluid that can be used for the research of Ethanol Consumption[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Endogenous metabolites is defined as those that are annotated by Kyoto Encyclopedia of Genes and Genomes as substrates or products of the ~1900 metabolic enzymes encoded in our genome. It is clear in the body of literature that there are documented toxic properties for many of these metabolites[1]. |
| References |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 291.3±40.0 °C at 760 mmHg |
| Melting Point | 304°C(dec.)(lit.) |
| Molecular Formula | C6H12O6 |
| Molecular Weight | 180.156 |
| Flash Point | 143.4±21.9 °C |
| Exact Mass | 180.063385 |
| PSA | 121.38000 |
| LogP | -2.11 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.784 |
| Storage condition | 2-8°C |
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2906199090 |
| HS Code | 2906199090 |
|---|---|
| Summary | 2906199090. cyclanic, cyclenic or cyclotherpenic alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Synthesis of allo- and epi-inositol via the NHC-catalyzed carbocyclization of carbohydrate-derived dialdehydes.
J. Org. Chem. 79(11) , 5088-96, (2014) A synthesis of carbocyclic sugars from carbohydrate-derived dialdehydes using organocatalysis has been developed. Sorbitol, mannitol, and galactitol were converted via 1,6-tritylation, perbenzylation ... |
|
|
Synthesis of phosphatidylinositols having various inositol stereoisomers by engineered phospholipase D.
J. Biosci. Bioeng. 109(4) , 337-40, (2010) Phospholipase D-mediated synthesis of phosphatidylinositols having various inositol stereoisomers was studied. Seven inositol stereoisomers were tested, all of which were found to be substrates of the... |
|
|
Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model.
Nat. Med. 12(7) , 801-8, (2006) When given orally to a transgenic mouse model of Alzheimer disease, cyclohexanehexol stereoisomers inhibit aggregation of amyloid beta peptide (Abeta) into high-molecular-weight oligomers in the brain... |
| 1,2,3,4,5,6-Cyclohexanehexol, (1α,2α,3α,4α,5α,6β)- |
| (1R,2R,3r,4S,5S,6s)-1,2,3,4,5,6-Cyclohexanehexol |
| (1R,2R,3r,4S,5S,6s)-Cyclohexane-1,2,3,4,5,6-hexol |
| epi-Inositol |
| 1,2,3,4,5/6-Inositol |
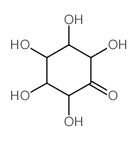 CAS#:488-64-2
CAS#:488-64-2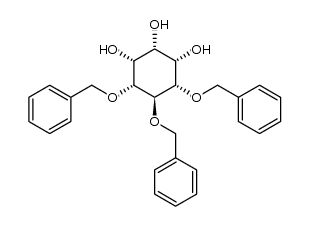 CAS#:190060-68-5
CAS#:190060-68-5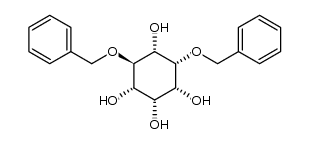 CAS#:283586-00-5
CAS#:283586-00-5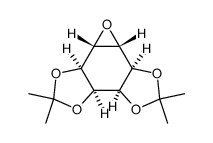 CAS#:744209-88-9
CAS#:744209-88-9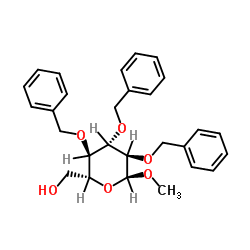 CAS#:53008-65-4
CAS#:53008-65-4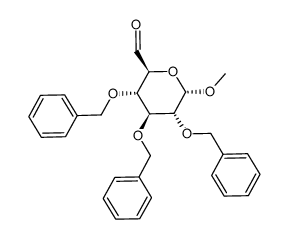 CAS#:83051-88-1
CAS#:83051-88-1 CAS#:4782-75-6
CAS#:4782-75-6
