1-Chlorohexadecane
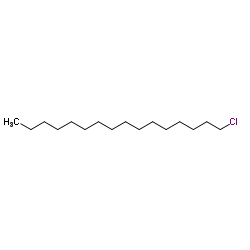
1-Chlorohexadecane structure
|
Common Name | 1-Chlorohexadecane | ||
|---|---|---|---|---|
| CAS Number | 4860-03-1 | Molecular Weight | 260.886 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 321.8±5.0 °C at 760 mmHg | |
| Molecular Formula | C16H33Cl | Melting Point | 8-14 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 138.1±3.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1-Chlorohexadecane |
|---|---|
| Synonym | More Synonyms |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 321.8±5.0 °C at 760 mmHg |
| Melting Point | 8-14 °C(lit.) |
| Molecular Formula | C16H33Cl |
| Molecular Weight | 260.886 |
| Flash Point | 138.1±3.4 °C |
| Exact Mass | 260.227081 |
| LogP | 8.94 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.446 |
| Water Solubility | 0.02 g/L (20 ºC, Dec.) |
| Freezing Point | 17.9℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| HS Code | 29031980 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 29031980 |
|---|
|
Haloalkane degradation and assimilation by Rhodococcus rhodochrous NCIMB 13064.
Microbiology 140 ( Pt 6) , 1433-42, (1994) The bacterium Rhodococcus rhodochrous NCIMB 13064, isolated from an industrial site, could use a wide range of 1-haloalkanes as sole carbon source but apparently utilized several different mechanisms ... |
|
|
Regiospecific internal desaturation of aliphatic compounds by a mutant Rhodococcus strain.
Appl. Environ. Microbiol. 65(12) , 5636-8, (1999) A mutant Rhodococcus strain lacking the ability to utilize 1-chlorohexadecane was found to cis-desaturate aliphatic compounds, such as 1-chlorohexadecane, n-hexadecane, and heptadecanonitrile, yieldin... |
|
|
Chlorinated fatty acid distribution in Mycobacterium convolutum phospholipids after growth on 1-chlorohexadecane.
Appl. Environ. Microbiol. 53(1) , 10-3, (1987) The composition of phospholipids from Mycobacterium convolutum R22 was determined after growth at two temperatures (20 and 30 degrees C) with 1-chlorohexadecane as the substrate. Comparisons were made... |
| 1-Cyclohexadecane |
| Palmityl chloride |
| 1-Chlorhexadecan |
| MFCD00000959 |
| 1-chloro-hexadecane |
| 1-CHLOROHEXADECYNE |
| 1-Hexadecylchlorid |
| chlorohexadecane |
| 1-hexadecane chloride |
| 1-chloro-hexadecan |
| EINECS 225-461-7 |
| CETYL CHLORIDE |
| hexadecanoyl chloride |
| 1-Chlorohexadecane |
| 1-Chlorohexadceane |
| Hexadecyl Chloride |
| Hexadecane, 1-chloro- |
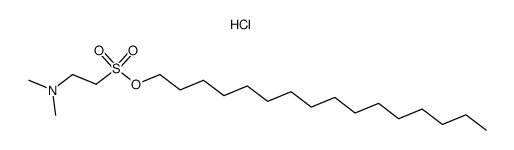 CAS#:66143-55-3
CAS#:66143-55-3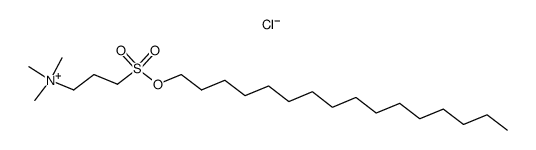 CAS#:66143-63-3
CAS#:66143-63-3 CAS#:54509-73-8
CAS#:54509-73-8 CAS#:36653-82-4
CAS#:36653-82-4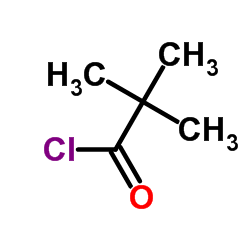 CAS#:3282-30-2
CAS#:3282-30-2 CAS#:26272-90-2
CAS#:26272-90-2 CAS#:37616-35-6
CAS#:37616-35-6 CAS#:83634-99-5
CAS#:83634-99-5 CAS#:3312-77-4
CAS#:3312-77-4 CAS#:4661-46-5
CAS#:4661-46-5 CAS#:110214-22-7
CAS#:110214-22-7 CAS#:38775-38-1
CAS#:38775-38-1 CAS#:506-12-7
CAS#:506-12-7 CAS#:21216-80-8
CAS#:21216-80-8 CAS#:18924-67-9
CAS#:18924-67-9 CAS#:22986-69-2
CAS#:22986-69-2 CAS#:26741-29-7
CAS#:26741-29-7 CAS#:629-73-2
CAS#:629-73-2 CAS#:27563-68-4
CAS#:27563-68-4
