N-Benzyladenosine
Modify Date: 2025-08-20 13:58:39
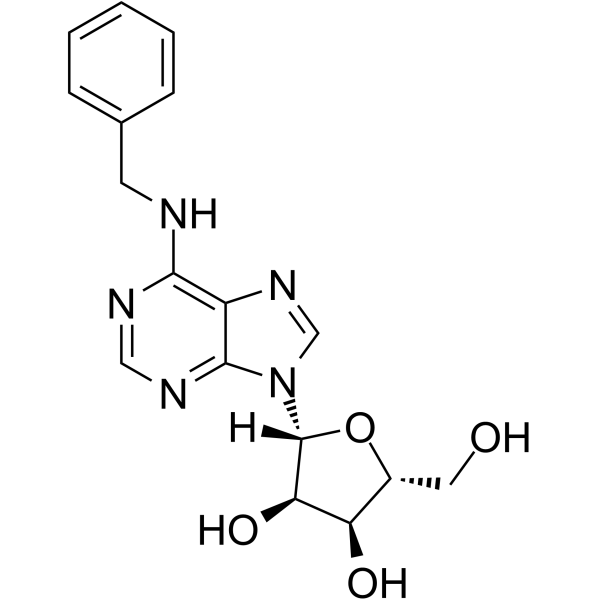
N-Benzyladenosine structure
|
Common Name | N-Benzyladenosine | ||
|---|---|---|---|---|
| CAS Number | 4294-16-0 | Molecular Weight | 357.364 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 689.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C17H19N5O4 | Melting Point | 184-186 °C | |
| MSDS | N/A | Flash Point | 370.7±34.3 °C | |
Use of N-BenzyladenosineN6-Benzyladenosine is an adenosine receptor agonist, has a cytoactive activity. N6-Benzyladenosine arrests cell cycle at G0/G1 phase and induces cell apoptosis. N6-Benzyladenosine also exerts inhibitory effect on T. gondii adenosine kinase and glioma[1]-[5]. |
| Name | n6-benzyladenosine |
|---|---|
| Synonym | More Synonyms |
| Description | N6-Benzyladenosine is an adenosine receptor agonist, has a cytoactive activity. N6-Benzyladenosine arrests cell cycle at G0/G1 phase and induces cell apoptosis. N6-Benzyladenosine also exerts inhibitory effect on T. gondii adenosine kinase and glioma[1]-[5]. |
|---|---|
| Related Catalog | |
| In Vitro | N6-benzyladenosine suppresses the clonogenic activity and the growth of different neoplastic cells[2]. N6-benzyladenosine results cell morphology alteration and actin cytoskeleton disorganization in T24 cell[2]. N6-benzyladenosine (10 μM; 24 h) is a potent inductor of apoptosis, and belongs to apoptotic systems with distinct caspase-3 and caspase-9 activation[3]. N6-benzyladenosine (0-100 μM; 24 h) induces chromatin condensation, formation of apoptotic bodies, and cleavage of DNA to nucleosomal fragments in a dose-dependent manner[3]. N6-benzyladenosine acts as a selective anti-toxoplasma agent with binding affinity to T. gondii adenosine kinase (apparent Km =179.8 μM), over human adenosine kinase[4]. N6-benzyladenosine (0-50 μM) shows weak inhibition against adenosine kinase deficient (TgAKS3) strains of Toxoplasma gondii[4]. N6-benzyladenosine (compound 2) (0.3-20 μM) exerts anti-glioma activity by interfering with the mevalonate pathway and inhibiting FPPS (Farnesyl pyrophosphate synthase) [5]. Apoptosis Analysis[3] Cell Line: HL-60 Concentration: 10 μM Incubation Time: 24 hours Result: Induced cell apoptosis by increasing caspase-3 (DEVDase) as well as caspase-9 (LEHDase) activity, indicating an apoptotic systems with distinct caspase-3/9 activation. Apoptosis Analysis[5] Cell Line: U87MG human glioma cell line. Concentration: 0.3, 0.6, 1.2, 2.5, 5, 10, 20 μM Incubation Time: 48 hours Result: Inhibited glioma growth by interfering with the mevalonate pathway and inhibiting FPPS. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 689.3±65.0 °C at 760 mmHg |
| Melting Point | 184-186 °C |
| Molecular Formula | C17H19N5O4 |
| Molecular Weight | 357.364 |
| Flash Point | 370.7±34.3 °C |
| Exact Mass | 357.143707 |
| PSA | 125.55000 |
| LogP | 1.04 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.763 |
| Storage condition | 2-8°C |
| Hazard Codes | Xn |
|---|---|
| Risk Phrases | 20/21/22-36/37/38 |
| Safety Phrases | S22-S24/25 |
| WGK Germany | 3 |
| Precursor 8 | |
|---|---|
| DownStream 7 | |
| Adenosine, N-(phenylmethyl)- |
| Adenosine, N-benzyl- |
| BAP RIBOSE |
| (2S,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol |
| N6-BENZYL-D-ADENOSINE |
| BAP RIBOSIDE |
| N-Benzyladenosine |
| 6-BENZYLADENOSINE |
| N6-Benzyladenosine |
| (4S,2R,3R,5R)-2-hydroxymethyl-5-[6-(benzylamino)-purin-9-yl]-tetrahydrofuran-3,4-diol |
| Adenosine, N- (phenylmethyl)- |
| Benzyladenosine |
| 6-Benzylaminopurine Ribosiden |
| EINECS 224-298-9 |
| N-(Phenylmethyl)adenosine |
| (-)-N6-(benzyl)adenosine |
| MFCD00005740 |
 CAS#:2004-06-0
CAS#:2004-06-0 CAS#:100-46-9
CAS#:100-46-9 CAS#:58-63-9
CAS#:58-63-9 CAS#:3287-99-8
CAS#:3287-99-8 CAS#:100-52-7
CAS#:100-52-7 CAS#:58-61-7
CAS#:58-61-7 CAS#:3181-38-2
CAS#:3181-38-2 CAS#:932-90-1
CAS#:932-90-1 CAS#:34408-14-5
CAS#:34408-14-5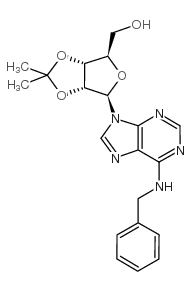 CAS#:78188-38-2
CAS#:78188-38-2 CAS#:2946-39-6
CAS#:2946-39-6 CAS#:1214-39-7
CAS#:1214-39-7 CAS#:18646-11-2
CAS#:18646-11-2 CAS#:13484-66-7
CAS#:13484-66-7