2-Methyl-L-proline
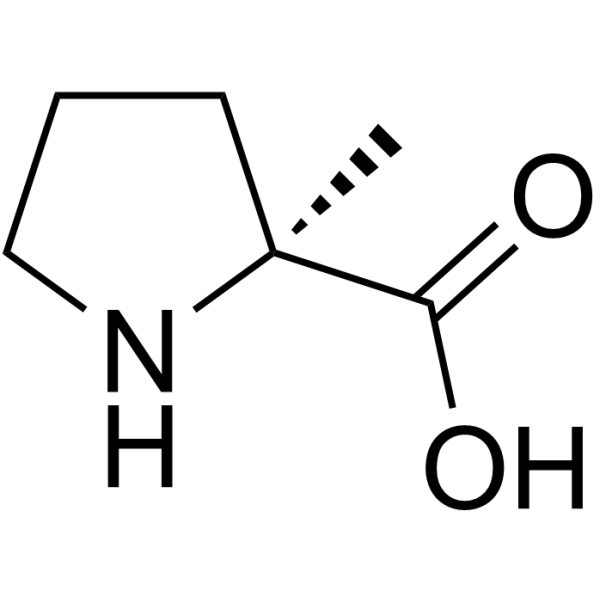
2-Methyl-L-proline structure
|
Common Name | 2-Methyl-L-proline | ||
|---|---|---|---|---|
| CAS Number | 42856-71-3 | Molecular Weight | 129.157 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 241.9±33.0 °C at 760 mmHg | |
| Molecular Formula | C6H11NO2 | Melting Point | 310 °C | |
| MSDS | Chinese USA | Flash Point | 100.1±25.4 °C | |
Use of 2-Methyl-L-proline(S)-2-Methylpyrrolidine-2-carboxylic acid is a proline derivative[1]. |
| Name | (S)-2-Methylpyrrolidine-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-2-Methylpyrrolidine-2-carboxylic acid is a proline derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 241.9±33.0 °C at 760 mmHg |
| Melting Point | 310 °C |
| Molecular Formula | C6H11NO2 |
| Molecular Weight | 129.157 |
| Flash Point | 100.1±25.4 °C |
| Exact Mass | 129.078979 |
| PSA | 49.33000 |
| LogP | -0.03 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.475 |
| Storage condition | -15°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 5 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effects of ring contraction on the conformational preferences of α-substituted proline analogs.
Biopolymers 98(2) , 98-110, (2012) The structural consequences derived from the incorporation of either a methyl or a phenyl group at the α carbon of proline were recently investigated by quantum mechanical calculations (J Org Chem 200... |
|
|
Beta-turns induced in bradykinin by (S)-alpha-methylproline.
FEBS Lett. 297(3) , 216-20, (1992) The ability of (S)-alpha-methylproline (alpha-MePro) to stabilise reverse-turn conformations in the peptide hormone bradykinin (BK = Arg1-Pro2-Pro3-Gly4-Phe5-Ser6-Pro7-Phe8-Arg9) has been investigated... |
|
|
alpha-Methylproline-containing renin inhibitory peptides: in vivo evaluation in an anesthetized, ganglion-blocked, hog renin infused rat model.
J. Med. Chem. 30(3) , 536-41, (1987) A structure-activity analysis of peptides containing backbone C alpha-methyl modification at the P4 site of the angiotensinogen sequence led to the discovery of potent renin inhibitors with apparent i... |
| MFCD01318647 |
| α-Methyl-L-proline |
| L-Proline, 2-methyl- |
| 2-Methyl-L-proline |
| (2S)-2-methylpyrrolidine-2-carboxylic acid,hydrochloride |
| (S)-2-Methyl proline |
 CAS#:132235-43-9
CAS#:132235-43-9 CAS#:848488-83-5
CAS#:848488-83-5 CAS#:86046-11-9
CAS#:86046-11-9 CAS#:6931-10-8
CAS#:6931-10-8![1-[(Benzyloxy)carbonyl]-2-methyl-L-proline Structure](https://image.chemsrc.com/caspic/447/63399-71-3.png) CAS#:63399-71-3
CAS#:63399-71-3 CAS#:1031890-09-1
CAS#:1031890-09-1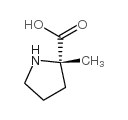 CAS#:63399-73-5
CAS#:63399-73-5![(1'R,2S,2'S,5'R)-1-[(2-isopropyl-5-methylcyclohexyl)oxymethoxy]-2-methyl-2-pyrrolidinecarbonitrile Structure](https://image.chemsrc.com/caspic/459/132235-36-0.png) CAS#:132235-36-0
CAS#:132235-36-0 CAS#:1268519-85-2
CAS#:1268519-85-2 CAS#:109837-32-3
CAS#:109837-32-3![(2S)-2-methyl-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid structure](https://image.chemsrc.com/caspic/131/103336-06-7.png) CAS#:103336-06-7
CAS#:103336-06-7 CAS#:869001-86-5
CAS#:869001-86-5
