UNII:Q2E57V2WS3
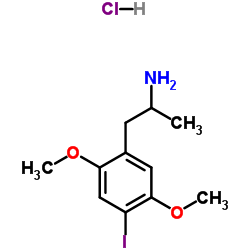
UNII:Q2E57V2WS3 structure
|
Common Name | UNII:Q2E57V2WS3 | ||
|---|---|---|---|---|
| CAS Number | 42203-78-1 | Molecular Weight | 357.616 | |
| Density | N/A | Boiling Point | 361.5ºC at 760 mmHg | |
| Molecular Formula | C11H17ClINO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 172.5ºC | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
| Name | (±)-DOI hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 361.5ºC at 760 mmHg |
|---|---|
| Molecular Formula | C11H17ClINO2 |
| Molecular Weight | 357.616 |
| Flash Point | 172.5ºC |
| Exact Mass | 356.999237 |
| PSA | 44.48000 |
| LogP | 3.70040 |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| HS Code | 2922299090 |
|
~% 
UNII:Q2E57V2WS3 CAS#:42203-78-1 |
| Literature: Glennon; Young; Benington; Morin Journal of Medicinal Chemistry, 1982 , vol. 25, # 10 p. 1163 - 1168 |
|
~69% 
UNII:Q2E57V2WS3 CAS#:42203-78-1 |
| Literature: Glennon; Young; Benington; Morin Journal of Medicinal Chemistry, 1982 , vol. 25, # 10 p. 1163 - 1168 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2922299090 |
|---|---|
| Summary | 2922299090. other amino-naphthols and other amino-phenols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex.
J. Pharmacol. Exp. Ther. 278 , 1373-1382, (1996) Correlations between 5-hydroxytryptamine (5-HT) receptor binding affinities and human hallucinogenic potency have suggested that 5-HT2 receptors mediate the hallucinogenic effects of lysergic acid die... |
|
|
Effect of the selective 5-HT receptor agonists 8-OHDPAT and DOI on behavior and brain biogenic amines of rats.
Gen. Pharmacol. 28 , 583-587, (1997) 1. The behavioral responses, as well as the biogenic amines and metabolite contents in discrete brain areas were determined in male rats subcutaneously treated with a 5-HT1A (8-OHDPAT) or 5-HT2A (DOI)... |
|
|
DOI, a 5-HT2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat striatum.
Brain Res. 698 , 204-208, (1995) The effects of (+-)-DOI (1-(2,5-dimethoxy-4-iodophenyl)-aminopropane) hydrochloride, a mixed 5-HT2A/2C receptor agonist, on the release of dopamine (DA) following D-amphetamine sulfate (AMP) or a DA D... |
| (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride |
| UNII:Q2E57V2WS3 |
| DOI hydrochloride,4-Iodo-2,5-dimethoxy-α-methylbenzeneethanaminehydrochloride |
| 1-(4-Iodo-2,5-dimethoxyphenyl)propan-2-amine hydrochloride (1:1) |
| 1-(4-Iodo-2,5-dimethoxyphenyl)-2-propanamine hydrochloride (1:1) |
| Benzeneethanamine, 4-iodo-2,5-dimethoxy-α-methyl-, hydrochloride (1:1) |
| (±)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride |

