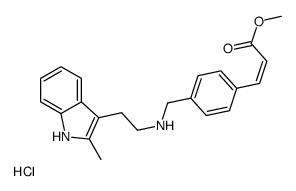
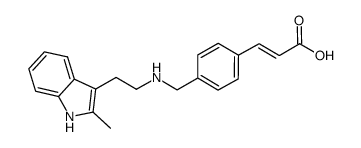
Panobinostat (LBH589)
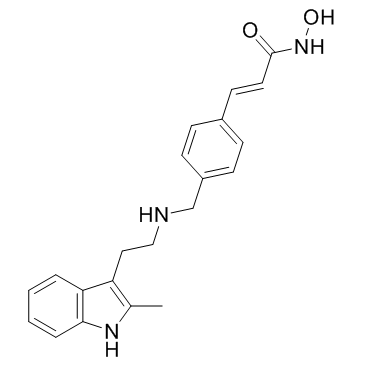
Panobinostat (LBH589) structure
|
Common Name | Panobinostat (LBH589) | ||
|---|---|---|---|---|
| CAS Number | 404950-80-7 | Molecular Weight | 349.426 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C21H23N3O2 | Melting Point | 114-117?C | |
| MSDS | N/A | Flash Point | N/A | |
Use of Panobinostat (LBH589)Panobinostat is a non-selective histone deacetylase (HDAC) inhibitor. |
| Name | panobinostat |
|---|---|
| Synonym | More Synonyms |
| Description | Panobinostat is a non-selective histone deacetylase (HDAC) inhibitor. |
|---|---|
| Related Catalog | |
| Target |
HDAC |
| In Vitro | Panobinosta (LBH589) induces apoptosis of both MOLT-4 and Reh cells in a time- and dose-dependent manner. Panobinosta treatment results in histone (H3K9 and H4K8) hyperacetylation and regulation of cell-cycle control genes in Reh cells[1]. Panobinostat exhibites potent antiproliferative activity in human NSCLC cell lines with the IC50 ranging from 5 to 100 nM[2]. |
| In Vivo | Panobinosta (10, 20 mg/kg, i.p.) significantly slows tumor growth derived from Meso and NSCLC cells in vivo models. Panobinosta markedly increases acetylation of histone H3 and H4 of H69 human SCLC cells harvest from SCID mice[2]. Panobinostat (5, 10 and 20 mg/kg i.p.) demonstrates a clear benefit of decreased tumor burden, significantly improves TTE and reduces bone density loss in a disseminated multiple myeloma mouse model[3]. |
| Cell Assay | Cells are washed with ice-cold PBS containing 0.1 mM sodium orthovanadate, and total proteins are isolated using RIPA lysis buffer, which includes protease inhibitors (leupeptin, antipain, and aprotinin), 0.5 mM PMSF, and 0.2 mM sodium orthovanadate. Protein amounts are quantified using the Bio-Rad protein assay. Equal amounts of proteins are loaded onto an sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred onto nitrocellulose membrane, and probed with the antibody of interest: mouse monoclonal c-Myc and mouse monoclonal p21 antibodies; rabbit polyclonal phospho-Histone H2A.X, rabbit polyclonal acetyl-Histone H3 (Lys9), and rabbit polyclonal acetyl-Histone H4 (Lys8) antibodies; mouse monoclonal p27/KIP1 antibody; mouse monoclonal anti-β-actin; and mouse monoclonal anti-GADD45G. Membranes are then washed, reprobed with appropriate horseradish peroxidase-conjugated secondary antibodies, and developed with SuperSignal chemiluminescent substrate. |
| Animal Admin | AE17 and TC-1 cancer cells (1×106 cells) are injected into the flanks of adult female C57Bl/6 mice and severe combined immunodeficiency (SCID) mice. M30 (10×106 cells), A549 (5×106 cells), H69 (2.5×106 cells), BK-T (6.5×106), H526 (10×106), and RG1 (10×106) cells are also injected, but in the presence of matrigel, into the flanks of SCID mice. When tumors reach 100 to 500 mm3, panobinostat is administered via i.p. injections (10-20 mg/kg) on a daily schedule (5-days-on, 2-days-off regimen) for the entire duration of the experiment. Control micereceive i.p. injections with dextrose 5% in water. Every tumor is measured with a caliper at least twice weekly. For evaluation of the effects of combination therapy on SCLC-derived tumors, SCID mice with H69 tumors are administered panobinostat. Three days after the initiation of panobinostat, and again 1 wk later, etoposide (40 mg/kg) is administered i.p. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 114-117?C |
| Molecular Formula | C21H23N3O2 |
| Molecular Weight | 349.426 |
| Exact Mass | 349.179016 |
| PSA | 77.15000 |
| LogP | 3.62 |
| Index of Refraction | 1.683 |
| InChIKey | FPOHNWQLNRZRFC-ZHACJKMWSA-N |
| SMILES | Cc1[nH]c2ccccc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |
| Storage condition | -20°C Freezer, Under Inert Atmosphere |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | 22-36/37/38-20 |
| Safety Phrases | 24/25 |
| HS Code | 2933990090 |
|
~% 
Panobinostat (L... CAS#:404950-80-7 |
| Literature: WO2007/21682 A1, ; Page/Page column 25-26 ; |
|
~% 
Panobinostat (L... CAS#:404950-80-7 |
| Literature: US2009/306405 A1, ; Page/Page column 5 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Panobinostat |
| (2E)-N-Hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide |
| (2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enamide |
| Farydak |
| LBH589 |
| 2-Propenamide, N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-, (2E)- |
| (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide |
| (E)-N-HYDROXY-3-(4-{[2-(2-METHYL-1H-INDOL-3-YL)-ETHYLAMINO]-METHYL}-PHENYL)-ACRYLAMIDE |
| (E)-N-hydroxy-3-(4-((2-(2-methyl-1H-indol-3-yl)ethylamino)methyl)phenyl)acrylamide |
| LBH-589 |
| (E)-N-Hydroxy-3-(4-(((2-(2-methyl-1H-indol-3-yl)ethyl)amino)methyl)phenyl)acrylamide |